All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Your opinion matters
At what grade are you comfortable prescribing ruxolitinib for your patients with steroid-refractory aGvHD?
Acute graft-versus-host disease (aGvHD) remains a prominent issue following hematopoietic stem cell transplants and presents an incidence and mortality rate of up to 50%. However, advancements in the prognosis and treatment of aGvHD have been a priority for the community. Iskra Pusic, Washington University School of Medicine, recently presented a summary of these advancements at the 4th Annual Meeting of the International Academy for Clinical Hematology (IACH).1 We summarize key points from this talk below.
Acute vs chronic GvHD
Pusic firstly outlined the differences between aGvHD, chronic GvHD (cGvHD), and overlap syndrome. These differences are detailed in Figure 1.
Figure 1. Timeline of acute and chronic GvHD onset post HSCT*
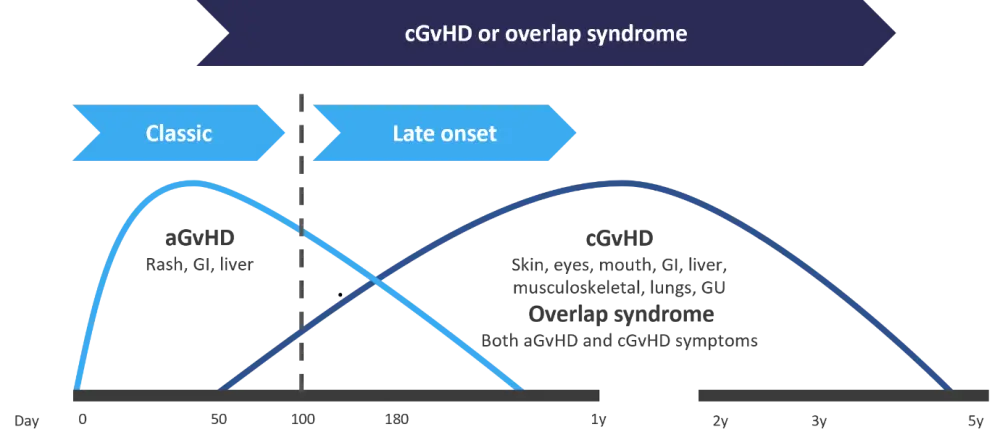
aGvHD, acute graft-versus-host disease; cGvHD, chronic graft-versus-host disease; GI, gastrointestinal; GU, genitourinary; HSCT, hematopoietic stem cell transplantation; y, year(s)
*Adapted from Pusic.1
aGvHD is diagnosed in the first 100 days post hematopoietic stem cell transplantation (HSCT) and manifests without any signs or symptoms of cGvHD. Late-onset aGvHD is diagnosed after Day 100 and occurs within 1 year post transplant, again without any cGvHD symptoms. Overlap syndrome occurs in patients who experience both symptoms of aGvHD and cGvHD. Classic cGvHD can occur at any time following HSCT, without any of the symptoms of aGvHD.
Current prophylaxis for acute GvHD
Multiple strategies of prophylaxis for aGvHD were highlighted in this talk. The current standard of care is the use of a calcineurin inhibitor, tacrolimus or cyclosporine, plus methotrexate. The immunosuppressant mycophenolate may be used in combination with sirolimus in patients unable to receive calcineurin inhibitors.
For prophylaxis following haploidentical HSCT, posttransplant cyclophosphamide is used as a backbone. Cyclophosphamide works by depleting alloreactive cells by eliminating proliferating cells and depleting intrathymic clonal T-cell precursors, while preserving regulatory T cells and exhausting effector T cells. Antithymocyte globulin has been used in trials based in the U.S for aGvHD and cGvHD following matched unrelated donor transplantation. Finally, T-cell depletion is currently an experimental method being investigated in clinical trials.
Prognosis
Pusic also outlined various factors used to assess aGvHD; describing the potential problems with various staging and grading criteria previously used to determine prognosis. She then discussed a prognostic system for aGvHD that utilizes clinical symptoms and compared this with a novel biomarker generated algorithm. Both of these forms of prognosis are designed to stratify patients by risk and, by extension, more accurately guide treatment strategies.
Using clinical symptoms
The Mount Sinai Acute GvHD International Consortium (MAGIC) consists of 25 transplant centers and uses a staging/grading system generated through standardized quantification of aGvHD symptoms. Gastrointestinal involvement is the primary driver of higher grading and severity of symptoms.
Treatment according to classification/grading of aGvHD varies, including the decision of whether to enroll in new clinical trials (Table 1).
Table 1. Treatment given according to the MAGIC grading system*
|
BSA, body surface area; CNI, calcineurin inhibitor; GI, gastrointestinal; GvHD, graft-versus-host disease; MAGIC, Mount Sinai Acute GvHD International Consortium. |
||||
|
Grade |
Symptoms |
Treatment |
Real-world treatment |
Clinical trials enrolment |
|---|---|---|---|---|
|
I |
≤50% BSA skin maculopapular rash No liver, upper GI, or lower GI involvement |
Continue/restart prophylaxis regimen + add topical steroids and supportive therapy |
Systemic steroids are given in 40% of cases |
No |
|
II–IV |
Erythroderma (>50% BSA) and liver, upper GI, and lower GI involvement of increasing severity with increased grade |
Standard of care is high dose steroids (1–2 mg/kg) Add topical steroids and supportive care |
Same as in clinical settings |
Yes, a priority |
|
Steroid-refractory GvHD |
Inadequate response to steroid + CNI after 5–7 days or progression after 3 days of therapy |
The only approved agent is ruxolitinib |
Ruxolitinib |
Yes, for experimental therapies including those targeting cytokine signaling pathways, e.g., JAK/STAT |
Another tool that adopts clinical symptoms is the Minnesota risk score, which was designed to identify patients at diagnosis who are at risk of developing steroid-refractory aGvHD (SR-aGvHD) or non-relapse mortality (NRM). In a validation of results, high-risk patients identified by this system were three times less likely to respond to steroids by Day 28 and had a greater than two-fold increase of risk of mortality.
Using biomarkers
Pusic also highlighted the work that the MAGIC consortium has begun with assessing serum biomarkers following transplant to determine NRM and GvHD.
Several biomarkers have been tested and the proteins ST2 and REG3α were identified and further investigated. These proteins enter the bloodstream from damaged gastrointestinal crypts. The levels of these two proteins are combined into a single value to produce a MAGIC algorithm probability (MAP) that can identify individual patient probability of 6-month NRM and overall survival.
Pusic presented a study that compared the accuracy of 6-month predictions for NRM, 28-day response, and overall survival using MAP versus clinical response for patients with SR-aGvHD. MAP was significantly more accurate over the first 4 weeks of prediction. Data were also presented to show that MAP is a better predictor of 6-month NRM when used 1 week after transplant compared with clinical response to therapy assessed at 4 weeks posttransplant.
The classification/grading of aGvHD lacks uniform standardization across clinics. However, evidence from the MAP system underlines the importance of integrating biomarkers in risk stratification and the predication of organ response to treatment, in turn producing more personalized therapy for patients with aGvHD.
Treatment of steroid-refractory aGvHD
Furthermore, Pusic discussed the treatment of SR-aGvHD, which is of particular importance owing to a high rate of NRM. Currently, the only therapy approved by the U.S Food and Drug Administration (FDA) for second-line treatment of aGvHD is the JAK1/2 inhibitor, ruxolitinib.
Two studies for ruxolitinib were highlighted in this talk; the REACH1 and REACH2 studies. The phase II REACH 1 study (NCT02953678), evaluating ruxolitinib in combination with corticosteroids, has demonstrated improved overall response in patients receiving allogeneic HSCT who develop SR-aGvHD; you can find the full results summarized here. The phase III REACH2 study (NCT02913261), which is also summarized on the GvHD Hub, has demonstrated improved partial and complete responses that were more sustained with ruxolitinib in patients with SR-aGvHD compared with best available therapy.
While ruxolitinib appears to be efficacious for this population, many other therapeutic avenues utilizing molecular targets in aGvHD pathophysiology have emerged. Other agents discussed by Pusic are summarized in Table 2.
Table 2. Experimental therapies for SR-GvHD*
|
aGvHD, acute graft versus host disease; cGvHD, chronic graft versus host disease; HSCT, hematopoietic stem cell transplantation; JAK, Janus kinase; mAb, monoclonal antibody; N/A, not applicable; SR, steroid-refractory. |
|||
|
Drug |
Mechanism of action |
Current status |
NCT number |
|---|---|---|---|
|
Cytokine targeting |
|||
|
Itacitinib |
JAK1 inhibitor |
Ongoing phase II/III GRAVITAS trial; itacitinib and corticosteroids as initial treatment for cGvHD |
|
|
Baricitinib |
JAK1/2 inhibitor |
Recruiting phase I trial for the treatment of GvHD after peripheral blood HSCT |
|
|
Alpha-1-antitrypsin |
Serum protease inhibitor that reduces inflammatory cytokines, particularly in gut |
Ongoing phase III trial investigating alpha-1-antitrypsin plus corticosteroids compared with corticosteroids alone |
|
|
Lymphocyte trafficking |
|||
|
Vedolizumab |
Humanized mAb; targets integrins and prevents trafficking of effector T cells to the gut |
Ongoing phase III trial investigating prophylactic use for intestinal aGvHD
|
|
|
Natalizumab |
α4 subunit adhesion molecule inhibitor |
Ongoing multicenter study on natalizumab plus standard steroid treatment for high risk aGvHD |
|
|
T-Guard |
Toxin-conjugated mAb targeting CD3/CD7 |
Recruiting phase III trial comparing T-Guard and ruxolitinib for the treatment of SR-aGvHD |
|
|
Cell regeneration and epithelial survival |
|||
|
IL-22 agonist |
Activation of IL-22 supports intestinal mucosa |
Preclinical research |
N/A |
Conclusion
Pusic highlighted the importance of advancements in aGvHD. She summarized promising changes, such as the introduction of a biomarker driven prognostic system to more accurately stratify aGvHD mortality risk and touched upon how biomarker stratification is now being used in clinical trial recruitment. Pusic also provided insight into the various molecular targets for therapeutic intervention in patients who are refractory to steroid treatment and how these may expand the therapeutic repertoire for aGvHD.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


 Iskra Pusic
Iskra Pusic