All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Abatacept as a novel agent for the treatment of steroid-refractory cGvHD: A phase II trial
The results of a phase II clinical trial of abatacept for steroid-refractory chronic graft-versus-host disease (cGvHD) were presented by Anita Koshy1 at the 2022 Transplantation & Cellular Therapy (TCT) Meetings of ASTCT and CIBMTR. Here, we summarize the study as presented at the TCT meetings.
Background and study aim
cGvHD represents a significant cause of morbidity and mortality following allogeneic transplantation, with a cumulative incidence of 50%. Steroids remain the mainstay of treatment; however, inadequate responses and potential toxicity fuel the search for longer lasting, more effective treatment.
Abatacept is a selective costimulation modulator that is a recombinant fusion protein consisting of the extracellular domain of human cytotoxic T-lymphocyte-associated antigen 4 linked to the modified Fc portion of human immunoglobulin G1. It binds to CD80 and CD86 on antigen presenting cells, reducing CD28-mediated T-cell activation and differentiation.
Following the promising results of the ABA2 trial (NCT01743131), in which abatacept was added to calcineurin inhibitor and methotrexate-based acute GvHD prophylaxis, the U.S. Food and Drug Administration (FDA) granted it priority review. Furthermore, a phase I trial of abatacept for the treatment of steroid-refractory cGvHD showed promising results with a clinical partial response of 44%, a 51.3% reduction in prednisone dose in responders, and no dose-limiting toxicity.
The aim of this study was to evaluate the overall response rate of abatacept in the treatment of steroid refractory cGvHD.
Study design and patient characteristics
The eligibility criteria and abatacept dosing are shown in Figure 1. Clinical assessment of cGvHD was performed using the National Institutes of Health Consensus Development Conference on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease, with each organ or site given a score from 0 to 3, with 0 reflecting no involvement and 3 reflecting severe impairment.
Figure 1. Study design*
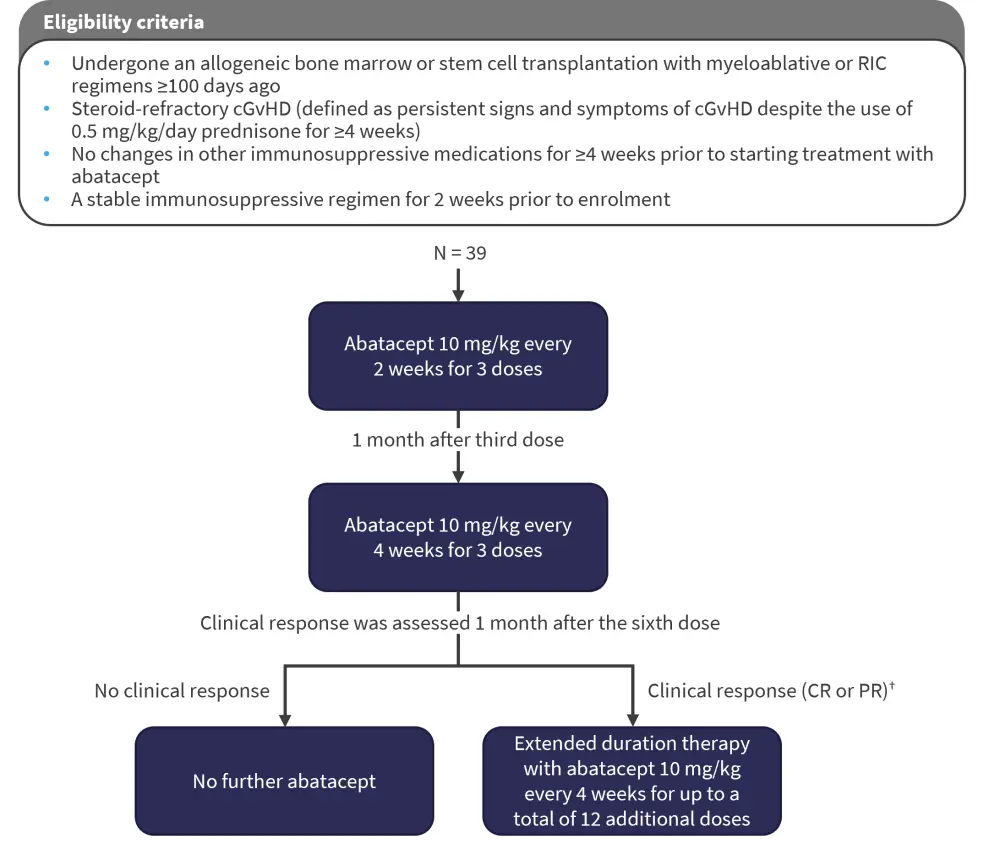
CR, complete response; PR, partial response; RIC, reduced intensity conditioning.
*Adapted from Koshy.1
†A CR was defined as resolution of all manifestations in each organ/site and PR was defined as improvement in ≥1 organ/site without progression in any others.
The characteristics of the study participants and transplant characteristics are shown in Table 1. In total, 39 patients were included in the study, with a median age of 62 years. Peripheral blood stem cells were the stem cell source in the vast majority of patients.
Table 1. Participant and transplant characteristics*
|
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BM, bone marrow; cGvHD, chronic graft-versus-host disease; CML, chronic myeloid leukemia; ECOG PS, Eastern Cooperative Oncology Group performance status; HLA, human leukocyte antigen; MDS, myelodysplastic syndromes; MPD, myeloproliferative disorders; NHL, non-Hodgkin lymphoma; MPN, myeloproliferative neoplasms; PBSC, peripheral blood stem cells. |
|
|
Characteristic, % (unless otherwise stated) |
N = 39 |
|---|---|
|
Participant baseline characteristics |
|
|
Median time from transplant to study entry (range), months |
43 (6–173) |
|
Median age (range), years |
62 (25–77) |
|
Sex |
|
|
Female |
53.8 |
|
Male |
46.2 |
|
ECOG PS |
|
|
0 |
2.6 |
|
1 |
69.2 |
|
2 |
28.2 |
|
Primary disease |
|
|
ALL |
12.8 |
|
AML |
46.2 |
|
CML |
5.1 |
|
MDS |
20.5 |
|
MPD |
5.1 |
|
NHL |
2.6 |
|
MPN with fibrosis |
2.6 |
|
Myelofibrosis |
2.6 |
|
cGvHD severity |
|
|
Moderate |
46.2 |
|
Severe |
53.8 |
|
Median prior treatments for cGvHD (range) |
5 (1–11) |
|
Most common sites of cGvHD involvement |
|
|
Skin |
84 |
|
Eyes |
72 |
|
Lung |
56 |
|
Joints |
82 |
|
Transplant characteristics |
|
|
Cell source |
|
|
BM |
10.3 |
|
PBSC |
89.7 |
|
Conditioning intensity |
|
|
Myeloablative |
61.5 |
|
Non-myeloablative |
35.9 |
|
HLA match |
|
|
Matched related |
38.4 |
|
Matched unrelated |
56.4 |
|
Mismatched related |
2.6 |
|
Mismatched unrelated |
2.6 |
Results
Patients received a median of eight doses of abatacept (range, 2–18). The overall response rate was 58% (complete response, 0%; partial response, 58%). The sites most improved were skin, mouth, eyes, joints, and lung (Table 2), and progression of disease occurred in 33% of patients; progression by site is shown in Table 2. A durable reduction in prednisone dose was also observed with abatacept treatment, with a 27.5% reduction at 1 month and a 50% reduction at 4 months follow-up (from 20 mg daily at study entry to 10 mg daily after 4 months).
Table 2. Clinical response or disease progression by organ site*
|
CR, complete response; PR, partial response. |
||
|
Organ/site of disease
|
Patients with CR or PR by organ/site, % |
Patients with progression of disease by organ/site, %† |
|---|---|---|
|
Skin |
22 |
6 |
|
Mouth |
22 |
14 |
|
Eyes |
25 |
3 |
|
Gastrointestinal tract |
8 |
— |
|
Liver |
17 |
3 |
|
Lung |
36 |
8 |
|
Joints |
22 |
6 |
Adverse events are shown in Table 3. Of note, there were nine events of neutropenia (Grades 2–4), which occurred in four patients.
Serious adverse events included two Grade 3 lung infections and one Grade 4 lung infection. One patient developed Grade 4 hemolysis, respiratory failure, hepatic failure, and subsequently died of concurrent herpes simplex virus hepatitis.
Table 3. Adverse events in patients treated with abatacept*
|
HSV, herpes simplex virus. |
|
|
Adverse event |
Number of events |
|---|---|
|
Common adverse events |
|
|
Neutropenia† |
|
|
Grade 2 |
5 |
|
Grade 3 |
2 |
|
Grade 4 |
2 |
|
Fatigue |
|
|
Grade 1 |
1 |
|
Grade 2 |
4 |
|
Headache |
|
|
Grade 2 |
4 |
|
Upper respiratory tract infection |
|
|
Grade 2 |
3 |
|
Grade 3 |
1 |
|
Serious adverse events |
|
|
Lung infection |
|
|
Grade 3 |
2 |
|
Grade 4 |
1 |
|
Hemolysis |
|
|
Grade 4 |
1‡ |
|
Respiratory failure |
|
|
Grade 4 |
1‡ |
|
Hepatic failure |
|
|
Grade 4 |
1‡ |
|
Death |
1‡ (from concurrent HSV hepatitis) |
Conclusion
This phase II trial of abatacept for the treatment of steroid-refractory cGvHD presented by Koshy at the 2022 TCT Meetings of ASTCT and CIBMTR showed a promising response rate with a durable reduction in prednisone dosing. Infusions were well tolerated, and severe infections were uncommon. Koshy highlighted that the group are performing ongoing immune correlative studies to better understand the mechanism of action of abatacept, including cytokine profiling of patient plasma and single-cell RNA sequencing to understand changes in the immune microenvironment. Flow cytometry has been used to assess interlukin-10 and interferon-γ expression in CD4+ T cells, with no difference observed before and after treatment in clinical responders.
Larger randomized trials are required to further assess the efficacy of abatacept and to clarify where it fits in the treatment pathway for cGvHD.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

