All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
A dose-ranging study of GvHD prophylaxis with PTB in patients with refractory acute leukemia
Patients with acute myeloid leukemia (AML) who are refractory to standard chemotherapy regimens have a poor prognosis. For patients with acute lymphoblastic leukemia (ALL), despite recent advances with novel targeted therapies, long-term outcomes remain poor due to relapse and resistance. Salvage allogeneic hematopoietic stem cell transplantation (HSCT) is a common option for these patients but is only curative in approximately 10–15% of patients, highlighting the need for novel, more efficacious transplantation approaches.
In a pre-clinical study, the substitution of post-transplant cyclophosphamide (PTCy) with post-transplant bendamustine (PTB) led to comparable graft-versus-host-disease (GvHD) control and significantly augmented graft-versus-leukemia (GvL) effect. However, the preventative effect of PTB alone and its optimal dosing are unknown. Moiseev et al.1 investigated PTB alone and in combination with other immunosuppressive agents for GvHD prophylaxis in patients with relapsed/refractory leukemia. The GvHD Hub is pleased to summarize the key findings here.
Study design1
A prospective, open-label, phase I/II dose-ranging study of PTB in patients with refractory acute leukemia (NCT02799147). Patients with AML, ALL, or mixed lineage acute leukemia were eligible if primary or secondary refractory to at least one course of induction or immunotherapy, had >5% of clonal blasts in bone marrow (BM) or peripheral blood, had an available sibling, 8–10/10 human leukocyte antigen (HLA) matched-unrelated, or haploidentical donor, and had a Karnofsky performance score (KPS) ≥70%.
All patients (N = 27) received myeloablative conditioning therapy (as shown in Figure 1) followed by allocation to either 70, 100, or 140 mg/m2 of PTB. The first 12 patients received only PTB, while the remaining 15 patients received a combination therapy with tacrolimus and mycophenolate mofetil (MMF) from Day +5. No other relapse prophylaxis therapy was permitted; however, pre-emptive therapy for measurable residual disease (MRD) was allowed.
- The primary endpoint was engraftment.
- Secondary outcomes included time to disease relapse, acute (a)GvHD, moderate-to-severe chronic (c)GvHD, non-relapse mortality (NRM), overall survival (OS), event-free survival (EFS), toxicity, and infectious complications.
Figure 1. Dosing schedule*
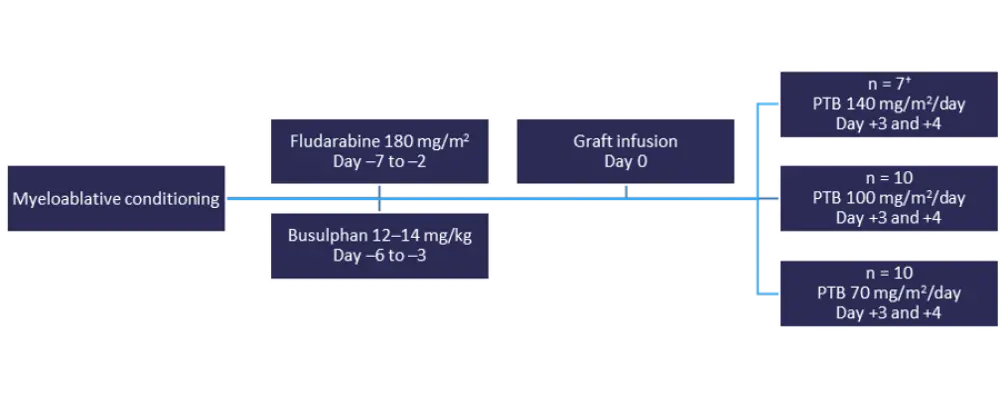
PTB, post-transplantation bendamustine.
*Adapted from Moiseev et al.1
†Enrollment halted at seven patients due to three consecutive cases of non-relapse mortality.
Results1
Baseline characteristics1
The median age of patients was 38 years (range, 20–56) and the median blast count in BM was 18% (range, 6–97) (as shown in Table 1). Of the patients, 22 had AML and five had ALL. At least one HLA-mismatch was seen in 15 donors, whereas the remaining 12 donors were 10/10 HLA-matched. The median KPS was 80% (range, 70–90), and 24 patients had a first allograft, two had a second, and one had a third allograft. Excess iron was seen in a significant number of patients at the time of HSCT.
Table 1. Baseline characteristics*
|
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BM, bone marrow; PBSC, peripheral blood stem cell. |
|
|
Characteristic |
N = 27 |
|---|---|
|
Male/Female, % |
48/52 |
|
Median age, years (range) |
38 (20–56) |
|
Diagnosis, % |
|
|
Graft source, % |
|
|
CD34+ cells, × 106/kg cells, mean ± SD |
6.2 ± 2.0 |
|
Refractory disease, % |
|
|
Previous induction therapies, median (range) |
2 (1–7) |
|
Median serum ferritin level, ng/mL (range) |
1,200 (48–3,828) |
|
Median blast count in BM, % (range) |
18 (6–97) |
|
Karyotype, % |
|
|
Donor, % |
|
Engraftment and cytokine release syndrome
- Engraftment was documented in 93% of the patients.
- The median time to white blood cell (WBC) engraftment and platelet engraftment was 17 and 14 days respectively, (range for both, 9–40 days). PBT-only prophylaxis showed significantly faster engraftment compared with PBT plus tacrolimus and MMF (median time to engraftment, 13 vs 20 days; p = 0.024).
- Similarly, peripheral blood stem cell (PBSC) grafts were also a significant predictor of faster engraftment compared with BM grafts (median time to engraftment, 16 vs 29 days; p = 0.002).
- An unusual complication, cytokine release syndrome (CRS) after PTB prophylaxis occured in 70% of patients (Grade 2 in 15%, Grade 3 in 26%, Grade 4 in 18%, and Grade 5 in 11%).
- The most common clinical presentation included high fever (67%), abnormal liver function tests (67%), pancreatitis (63%), skin vasculitis (56%), enterocolitis (48%), oral mucosal inflammation (37%), disseminated intravascular coagulation (37%), and central nervous system toxicity (26%).
- CRS occurrence was more significant with HLA-mismatched donors (75% vs 20%; p = 0.004) and haploidentical donors (88% vs 42%; p = 0.030).
No differences were observed in the incidence of severe CRS (p = 0.795) or in severity of CRS (p = 0.642) between patients receiving PTB alone or in combination with tacrolimus and MMF.
OS, NRM, and relapse incidence
In total, 89% patients achieved a complete response (CR) and 63% had MRD-negative remission. A higher proportion of the patients with AML (71%) were MRD negative compared with patients with ALL (40%). Table 2 shows the OS, EFS, NRM, and relapse incidence for all patients.
- There were no significant differences between the different dose groups (p = 0.35). However, survival was higher in the PTB 100 mg/kg2 group, with 3-year OS estimates of 14%, 40%, and 26% for dose levels 140 mg/kg2, 100 mg/kg2, and 70 mg/kg2, respectively.
- Survival was also higher in patients with AML compared with patients with ALL (35% vs 0%; p = 0.16). The disease was not effectively controlled with the regimen in patients with ALL, all but one patient died from disease progression.
- No late relapses were observed beyond Day 100, indicating a persistent GvL effect.
Table 2. OS, EFS, NRM, and relapse incidence*
|
AML, acute myeloid leukemia, CI, confidence interval; EFS, event-free survival; NRM, non-relapse mortality; OS, overall survival. |
|
|
Outcome |
Patients, % (95% CI) |
|---|---|
|
3-year OS |
28 (13–46) |
|
EFS |
29 (13–46) |
|
NRM |
46 (25–64) |
|
Cumulative incidence of relapse |
26 (11–44) |
Adverse events and toxicities
The adverse events (AEs) were as expected for patients with AML and ALL (see Table 3). The most common causes of mortality were progressive disease (22%) and CRS-related deaths (22%).
- The cumulative incidence of aGvHD was 44% and moderate to severe cGvHD was 56%. Grade 3–5 CRS was associated with Grade II–IV aGvHD (25% vs 67%; p = 0.031).
- 100-day survival was higher in the PTB-only group compared with PTB in combination with other immunosuppressive agents (100% vs 18%; p = 0.002).
Table 3. Adverse events*
|
AE, adverse event; CMR, cytomegalovirus reactivation; TATM, transplant-associated thrombotic microangiopathy. |
|
|
Grade 3–4 AEs, % |
Patients |
|---|---|
|
Mucositis |
30 |
Conclusion
This study demonstrates that PTB could be a potential GvHD prophylactic treatment for supplementing the GvL effect in refractory AML. The complications of developing severe cGvHD after single-agent PTB therapy with the use of HLA-matched allografts can be controlled with a combination of PTB with tacrolimus and MMF starting on Day +5. However, PTB cannot be used without additional immunosuppression even in the low-risk GvHD population, such as recipients of matched-related BM transplantation, due to high rate of CRS.
Future studies are therefore needed to investigate CRS and effectiveness of PTB in a larger sample using patients with ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

