All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Targeting the ICOS-ICOSL costimulatory pathway in gastrointestinal aGvHD
Gastrointestinal graft-versus-host disease (GI-GvHD) is associated with high mortality, consequently, identifying cellular biomarkers for disease monitoring and drug targeting is an area of active research. The development of T helper 17 (Th17) cells is reliant upon the interaction between the inducible costimulator (ICOS) receptor and its ligand (ICOSL) on dendritic cells (DCs). A particular pathogenic T-cell population that produces IL-17 and expresses CD4+CD146+CCR5+ is known to be increased in the blood of patients with GI-GvHD, and it is considered that the ICOSL on DCs may provide a stimulus for generating these T cells in patients.
In a study published in Science Translational Medicine, Djamilatou Adom et al. evaluated the importance of the ICOS–ICOSL pathway in acute (a)GvHD using ICOSL-knockout mice, and the effect of treatment with a dual ICOS/CD28 inhibitor in humanized murine models of aGvHD.1 Here we summarize their findings.
Study design
The study was performed in three parts. Part 1 was to investigate the expression of ICOSL on plasmacytoid DCs (pDCs) of 156 patients receiving hematopoietic cell transplant (HCT). The study population consisted of 64 patients with GI-GvHD, 31 with skin GvHD, 22 with non-GvHD enteritis, and 39 without GvHD. Peripheral blood mononuclear cells (PBMCs, collected weekly for the first 4 weeks and then monthly after allogeneic HCT) were analyzed by multiparametric flow cytometry using panels of T-cell and DC markers.
In Part 2, to characterise intestinal CD146+CCR5+ T cells and pDCs, the investigators firstly used an aGvHD major mismatch HCT murine model in which recipients were given either ICOSL-deficient bone marrow (BM), wild-type BM, or STAT3-deficient BM. Secondly, a xenogeneic aGvHD model was used, whereby human PBMCs were engrafted into immunodeficient NOD SCID gamma (NSG) mice. In this model, pDCs were stimulated to increase expression of ICOSL, mixed with pDC-depleted human PBMCs, and transplanted into the NSG mice. T cells and DCs were characterized by flow cytometry.
Part 3 was to assess the therapeutic potential of a dual ICOS/CD28 inhibitor, ALPN-101, in vitro, using human T cell lines, and in vivo, using the human PBMC-NSG GvHD model. ALPN-101 dosing schedules are described in the results below. The performance of ALPN-101 was compared to that of belatacept, a CD28/CTLA-4 pathway inhibitor in clinical use. Disease activity scoring incorporated overall health and activity, skin and hair changes, and body weight loss.
Results
Part 1: ICOSL+ pDC frequency is a potential biomarker for GI-GvHD
- A higher frequency of ICOSL+ pDCs was found in patients with GI-GvHD (24.1%), compared with patients without GvHD (4.6%), or patients with non-GvHD enteritis (5.7%) or skin GvHD (5.6%)
- The frequency of ICOSL+ pDCs correlated with the number of CD4+CD146+CCR5+ T cells in all symptomatic patients (non-GvHD enteritis/skin GvHD/GI-GvHD) as well as in patients with GI-GvHD only.
- By receiver operating characteristic analysis, the combination of CD4+CD146+CCR5+ T cells and ICOSL+ pDC frequencies was a higher predictor for GvHD (area under curve [AUC]: 0.80) and GI-GvHD (AUC: 0.88) than either biomarker alone (for GvHD and GI-GvHD, respectively, AUC: 0.78 and 0.75 for CD4+CD146+CCR5+ T cells, and 0.71 and 0.83 for ICOSL+ pDCs).
- Using the median frequency of ICOSL+ pDCs (8.23%) in patients with symptomatic GvHD as a threshold for mortality risk, a high frequency of ICOSL+ pDCs was significantly associated with a lower 3-year overall survival (p = 0.004).
Taken together, the results indicate that ICOSL+ pDC frequencies may serve as an additional GI-GvHD biomarker and support the notion that ICOSL+ pDCs cell numbers correlate with the frequency of CD4+CD146+CCR5+ T cells in patients with GI-GvHD.
Part 2: Role of ICOSL signaling in aGvHD
In a major mismatch HCT murine model, recipients of ICOSL-deficient BM had lower GvHD scores (p < 0.0001) and superior survival (p < 0.0001), compared with mice receiving wild-type BM, or BM deficient in STAT3, a transcription factor required for the development of DC precursors in mice. Compared with recipients of wild-type BM, ICOSL-deficient BM recipients also had:
- reduced levels of the FLT3 ligand (a growth factor necessary for DC activation) in the serum 3 days after HCT (p = 0.01)(Similar reductions in FLT3 ligand concentrations were observed in a haploidentical experimental model, indicating that the results were not mouse-strain specific),
- reduced intestinal infiltration of pDCs and Th17 cells (p < 0.001 for absolute number of pDCs and IL-17+/IFNγ+ T cells at Day 14 after HCT) (similar results were observed in the haploidentical experimental model),
- reduced frequency and absolute number of splenic pDCs and Th17 cells,
- intestinal pDCs with lower expression of genes encoding molecules that are essential for pDC development at Day 14 after HCT, and
- effector T cells with decreased expression of T-cell activation markers, including Prf1, Emoes, Il17a, CD28, Gzmb, Ifng, and Icos, at Day 14 after HCT.
In the human PBMC-NSG GvHD model,
- recipients of ICOSL+ pDCs had more severe GvHD (p = 0.0028) and inferior survival (p = 0.0021), compared with mice receiving unstimulated pDCs, and
- frequencies of human CD45+ and human Th17 cells in both the spleen and intestines were increased in the recipients of ICOSL+ pDCs, compared with recipients of unstimulated pDCs.
Part 3: Dual ICOS/CD28 inhibition supresses T cell responses in vitro
A dual ICOS and CD28 antagonist (ALPN-101) was developed as a potential therapeutic using the variant immunoglobulin domain (vIgD) platform. ALPN-101 consists of the ICOSL IgV domain fused to a human IgG1 Fc fragment (which binds FcRn but not FcγR or complement C1q). Figure 1 compares the dual mechanism of action of ALPN-101 to that of a CTLA-4-Fc fusion protein.
Figure 1. Schematic representation of the mechanism of action for ALPN-101 and CTLA-4-Fc fusion protein1
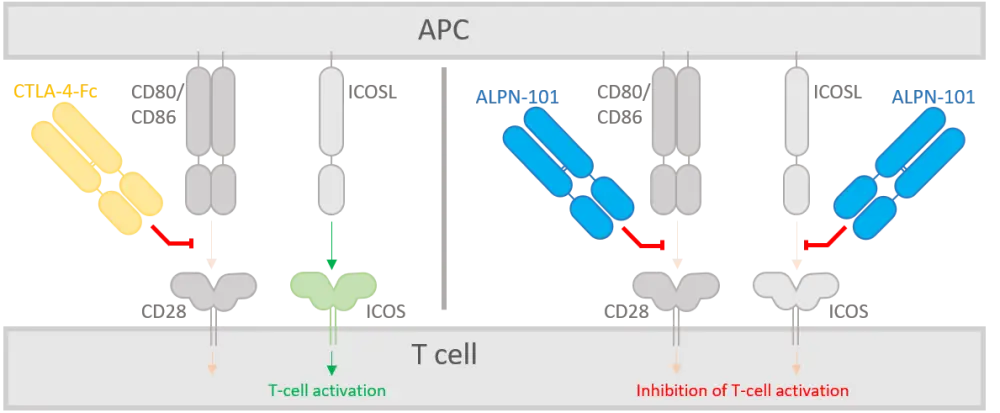
APC, antigen-presenting cell; ICOS, inducible costimulator receptor; ICOSL, ICOS ligand.
Results showed potent inhibition of both ICOS and CD28 signaling pathways in vitro:
- In a human Jurkat T cell line, ALPN-101 inhibited ICOS-mediated signaling as effectively as an anti-ICOSL monoclonal antibody positive control, and inhibited CD28-mediated signaling more potently than the two approved CTLA-4-Fc fusion proteins, abatacept and belatacept.
- ALPN-101 reduced the proliferation of CD4+ and CD8+ effector T cells and inhibited production of effector T cell cytokines (IFNγ, IL-2, IL-17A, IL-13, granulocyte-macrophage colony stimulating factor, and TNFγ).
Part 3: Dual ICOS/CD28 inhibition prevents aGvHD in vivo
In studies using the human PBMC-NSG GvHD model, the investigators found that 12 injections of ALPN-101 (at doses of either 20 µg, 100 µg, or 500 µg) prevented xenogeneic aGvHD, compared with saline-injected controls (100% vs 0% survival, p < 0.001). A single dose of 100 µg of ALPN-101 resulted in:
- similar protection from xenogeneic aGvHD as repeat doses of belatacept (12 × 100 µg doses over 4 weeks), with survival rates of 25% for single-dose ALPN-101 vs 40% for repeat-dose belatacept,
- superior survival, compared with saline treatment (p < 0.001), and
- significantly lower disease activity index (DAI) scores compared with saline treatment (p < 0.0001), but similar DAI scores to repeat-dose belatacept (p = 0.3625).
Compared with repeated doses of belatacept, mice receiving repeat doses of ALPN-101 had reduced DAI scores at all dose levels (p = 0.002 for 500 µg doses and p = 0.011 for 20 µg doses). Consistent with improved overall health, the body weight of mice receiving repeat doses of ALPN-101 increased over the course of the experiment. Body weight for single-dose ALPN-101 and repeat-dose belatacept groups was maintained.
Blood from surviving mice was analyzed 2 weeks after the last treatment with repeated doses of ALPN-101 or belatacept.
- Flow cytometric analysis of the human T cells showed the absence of CD28 and ICOS signals, confirming the on-target effect of ALPN-101.
- Repeat-dose ALPN-101 treatment reduced the serum levels of inflammatory cytokines, including IFNγ and TNFα, more effectively than saline or repeat-dose belatacept, whereas single-dose ALPN-101 showed no protective effect.
The activity of ALPN-101 was compared to that of independent anti-ICOS and anti-CD28 fusion proteins in Jurkat cell lines expressing endogenous CD28 and transfected ICOS-CD28 molecules. ALPN-101 was bound to, and inhibited stimulation of, the Jurkat cells more effectively than either ICOS or CD28 fusion proteins alone.
Part 3: Prophylactic ALPN-101 extends survival in a xenogeneic aGvHD model
A modified xenogeneic aGvHD model, in which mice were monitored for 85 days after HCT, was used to evaluate the effectiveness of a prophylactic treatment regimen.
- Whilst no mice survived to Day 85 in groups receiving a prophylactic regimen of cyclosporin A (CsA; 20 mg/kg daily for Days –1 to +13, then three times weekly for 4 weeks) or repeated-dose belatacept, a single 500 µg dose of ALPN-101 increased survival to 50%.
- Improved health following ALPN-101 injection was also reflected by higher body weight and lower clinical scores. Further improvement of clinical scores could be achieved by combining ALPN-101 with CsA.
- The frequencies of CD4+ and CD8+ effector T cells were reduced, whereas regulatory T-cell suppressive activity was not impacted in the ALPN-101-treated mice.
In another modified xenogeneic NSG model of aGvHD, mice received a prophylactic dosing schedule of either 100 µg ALPN-101 every other day from Day –1 to +21, or two doses of ALPN-101 on Day –1 and +1.
- Increased body weight and lower GvHD scores, compared with control recipients, indicated protection against xenogeneic aGvHD with either schedule of prophylactic ALPN-101.
- Superior survival was also observed; most mice that received ALPN-101 survived to 60 days, whereas only one mouse in the control group survived beyond 40 days.
- There was reduced intestinal and splenic infiltration of human CD45+ cells, DCs, and Th17 cells in ALPN-101 treated mice.
Prophylactic ALPN-101 also conferred protection against xenogeneic aGvHD in a more aggressive NSG model, where mice were irradiated with 350 cGy and given 10 mg human IgG and 5 × 106 PBMCs. Taken together these data suggest that ALPN-101 could be an effective prophylactic therapy option.
Part 3: Treatment of aGvHD with ALPN-101 improves symptoms and outcome
The same aggressive NSG model was used to assess whether ALPN-101 initiated at the onset of disease signs could provide an effective aGvHD treatment option.
- Lower clinical scores, reduced weight loss, and improved survival was observed in mice treated with ALPN-101 (either 20 µg or 100 µg doses at disease onset and every other day from Day +7 to +14), compared with controls.
- Human CD45+ cells, DCs, and Th17 cells were almost undetectable in the intestines of ALPN-101 treated mice.
To determine whether ALPN-101 treatment would preserve antitumoral activity whilst reducing aGvHD, the authors evaluated the impact of ALPN-101 on the survival of mice with acute myeloid leukemia. Mice treated with ALPN-101 after PBMC transplant had superior survival to (a) those receiving no PBMC transplant or ALPN-101 treatment, (b) those receiving ALPN-101 but no transplant, and (c) those receiving PBMC transplant and Fc control treatment.
Conclusion
In this comprehensive analysis, the authors confirmed a positive association between ICOSL expression on pDCs and GI-GvHD, and propose that ICOSL+ pDC frequencies may be used as an early biomarker to identify patients at risk of GI-GvHD who may benefit from intervention.
The use of ICOSL-deficient BM as well as therapeutic inhibition of both ICOS and CD28 signaling using a dual-targeted antagonist, ALPN-101, conferred protection against aGvHD. Dual targeting proved more potent, even with a single dose, than single inhibition of the CD28/B7 or ICOS–ICOSL pathways.
The preclinical in vivo studies suggest that ALPN-101 has the potential to both prevent and treat aGvHD whilst maintaining antileukemic activity. Clinical trials are required to further evaluate the therapeutic potential of ALPN-101.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

