All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Safety and efficacy of axatilimab in patients with cGvHD who have failed ≥2 lines of systemic therapy
Do you know... The SNDX-6352-0503 phase I/II trial (NCT03604692) investigated the safety and efficacy of axatilimab (axa) in patients with active cGvHD who had failed ≥2 prior lines of therapy. Phase I of the study evaluated escalating dose levels of axa to establish the recommended phase II dose (RP2D). Identify the correct RP2D from the list below.
Chronic graft-versus-host disease (cGvHD) is a major cause of late morbidity and mortality in an estimated 30–50% of patients receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT).1 Corticosteroids are standard frontline treatment, but nearly 50% of patients with cGvHD need second-line treatment for disease progression or inadequate response. Although ibrutinib, ruxolitinib, and belumosudil have been approved by the U.S. Food and Drug Administration (FDA) for cGvHD, many patients still fail to respond and eventually progress. Axatilimab (also known as SNDX‑6352), an immunoglobulin G4 monoclonal antibody, blocks colony stimulating factor-1 receptor-expressing monocytes and targets profibrotic macrophage-driven diseases such as cGvHD.1
During the 2022 Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR, Carrie Kitko1 presented the results of the SNDX-6352-0503 phase I/II trial (NCT03604692) investigating the safety and efficacy of axatilimab in patients with active cGvHD who had failed ≥2 prior lines of therapy. The key findings are summarized here.
Study design
This was a phase I/II study in patients aged ≥6 years with active cGvHD who failed ≥2 prior lines of systemic therapy and had a Karnofsky performance status ≥60. Phase I of the study evaluated escalating dose levels of axatilimab at 0.15 mg/kg, 0.5 mg/kg, 1 mg/kg, and 3 mg/kg to establish the recommended phase II dose (RP2D). The phase II dose expansion evaluated axatilimab at 1 mg/kg (Figure 1).
Figure 1. Treatment schema*
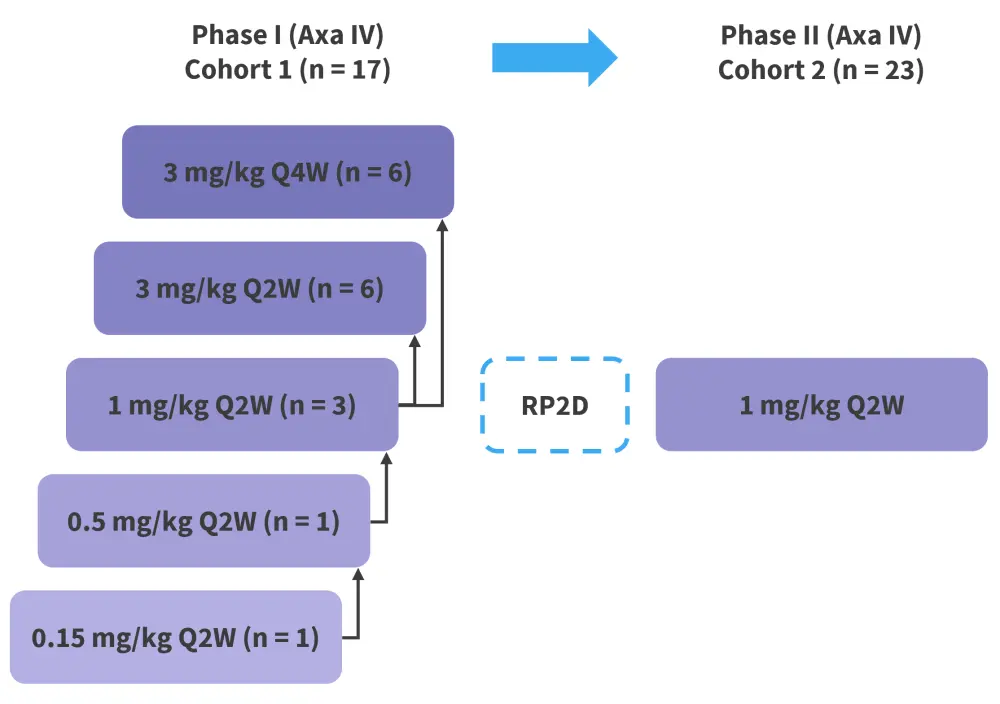
Axa, axatilimab; IV, intravenous; Q2W, once every two weeks; Q4W, once every 4 weeks; RP2D, recommended phase II dose.
*Adapted from Kitko.1
The primary endpoint in the phase II expansion was overall response rate (ORR; complete response + partial response) at 6 months. Secondary outcomes included duration of response defined by the 2014 National Institutes of Health (NIH) Consensus Development Project on criteria for clinical trials in cGvHD, adverse events (AEs), and organ-specific response rate.2
Results
A total of 40 (phase I, n = 17; phase II, n = 23) patients were included and received at least one dose of axatilimab. Patient characteristics were not significantly different between the phase I and II portions of the study. In total, 65% of patients had received myeloablative transplantation and 45% had related donors. Other patient characteristics are shown in Table 1. At data cut-off, 23 patients had discontinued treatment.
Table 1. Baseline characteristics*
|
C1D1, Cycle 1 Day 1; cGvHD, chronic graft-versus-host disease; PS, performance status. |
|
|
Characteristics |
All patients (N = 40) |
|---|---|
|
Median age (range), years |
59 (16–73) |
|
Male, % |
63 |
|
Median Karnofsky PS (range) |
80 (60–100) |
|
Median duration of cGvHD prior to C1D1 (range), years |
3.2 (0.11–15.6) |
|
Prior lines of therapy |
|
|
Median (range) |
4 (1–11) |
|
Ibrutinib, % |
65 |
|
Ruxolitinib, % |
53 |
|
Belumosudil, % |
20 |
|
Organ involvement at baseline |
|
|
Median (range) |
4 (1–9) |
|
≥4 organ involvement, % |
65 |
Efficacy
Responses observed in patients were rapid and durable, particularly even at low doses. The ORR was 68% amongst 31 evaluable patients treated at a dose of 1 mg/kg every 2 weeks (Q2W) and 3 mg/kg every 4 weeks (Q4W) (doses advancing to the pivotal AGAVE-201 trial [NCT04710576]). The median time to response was 0.9 month (range, 0.9–11 months) and the median duration of treatment was 6.7 months (range, 0.9–26.7 months) across all cohorts. Organ responses in difficult-to-treat organs, such as joints, fascia, and lungs, are shown in Figure 2. At baseline, 81% of patients had severe skin sclerosis. After treatment, 16% showed improvement of their sclerosis meaning they had complete reversal of all the sclerotic manifestations as defined by the NIH criteria.
Figure 2. Best response in difficult-to-treat organs*
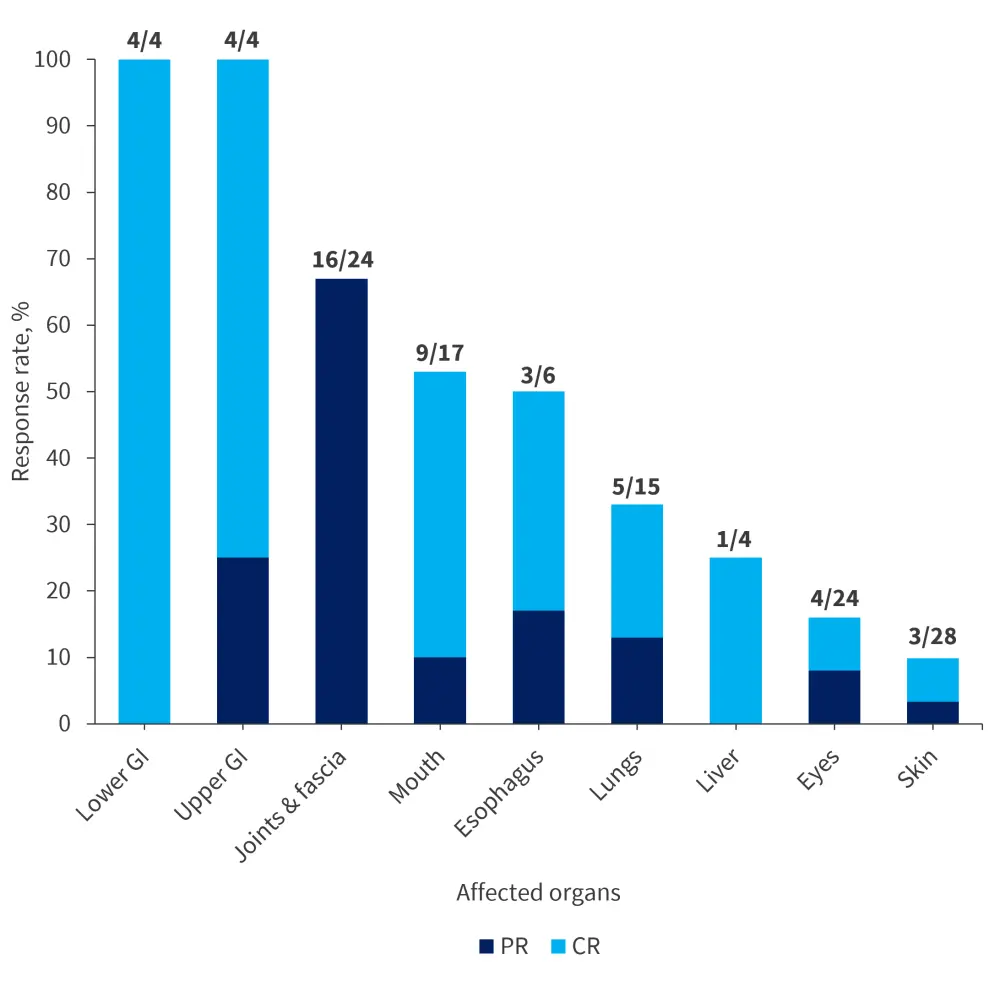
BR, best response; CR, complete response; GI, gastrointestinal tract; PR, partial response.
*Adapted from Kitko.1
Safety
At data cut-off, 17 (43%) patients continued and 23 (58%) patients discontinued treatment. Reasons for discontinuation included disease progression (18%), physician decision (15%), AEs (10%), and patient decision (5%). In the overall cohort, 73% of patients experienced treatment-emergent adverse events (TEAEs). Of the patients treated with doses 1 mg/kg Q2W and 3 mg/kg Q4W, 20% experienced Grade ≥3 AEs. These included three cases (8%) of elevated creatine phosphokinase, two cases (5%) of increased lipase, and one case each (3%) of hypersensitivity, periorbital edema, and septic arthritis. Regardless of their grades, TEAEs showed a trend towards dose dependency (1 mg/kg Q2W and 3 mg/kg Q4W), with a higher proportion of patients having elevations in aspartate aminotransferase, creatinine phosphokinase, alanine aminotransferase, and lipase (Table 2).
The elevated serum enzyme level reflected the target effect of axatilimab on Kupffer cells in the liver and was not associated with end-organ damage. Regardless of the NIH criteria, 53% (16/30) of patients achieved a 7-point reduction from baseline in normalized Lee Symptom Scale (LSS). The median change in LSS was −7.8 (range, 3.1 to −37.6). The risk of infection was low and comparable to other cGvHD trials. There were no cases of viral reactivation reported (cytomegalovirus, Epstein-Barr, and/or herpes simplex virus).
Table 2. Safety outcomes*
|
TEAEs, treatment-emergent adverse events. |
|
|
TEAEs, % |
All patients (N = 40) |
|---|---|
|
All TEAEs |
73 |
|
Aspartate aminotransferase increased |
35 |
|
Creatine phosphokinase increased |
33 |
|
Alanine aminotransferase increased |
25 |
|
Lipase increased |
23 |
|
Amylase increased |
23 |
|
Infection |
48 |
|
Upper respiratory infection |
18 |
|
Cellulitis |
10 |
|
Pneumonia |
5 |
|
Pseudomonas infection |
5 |
|
Urinary tract infection |
5 |
|
Influenza |
5 |
Conclusion
This phase I/II study demonstrated a tolerable safety profile and efficacy of axatilimab in a population that was heavily pretreated with ≥2 prior lines of systemic therapies and had active cGvHD. An ORR of 68% was achieved and 43% of patients were continuing treatment as of data cutoff. Findings from this study will inform the pivotal trial AGAVE-201 that has started enrolling patients, evaluating the efficacy of three doses of axatilimab (1 mg/kg Q2W, 3 mg/kg Q4W and 0.3 mg/kg Q2W).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?



