All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Positive interim findings from the EQUATE trial, including the mechanism of action of itolizumab in patients with aGvHD
Acute graft-versus-host disease (aGvHD) occurs after allogeneic hematopoietic stem cell transplantation (allo-HSCT) and is a leading cause of mortality. The CD6-activated leukocyte cell adhesion molecule (ALCAM) pathway plays an important role in inflammation and autoimmunity.1 Earlier studies have demonstrated that depletion of CD6+ T cells prior to allo-HSCT decreases the incidence of aGvHD, suggesting CD6 is a target relevant to aGvHD pathogenesis.2
Itolizumab, a humanized immunoglobulin G1 (IgG1) anti-CD6 monoclonal antibody, inhibits CD6 signaling, consequently decreasing T-cell activation and proliferation2. Itolizumab is currently being investigated in the EQUATE trial (NCT03763318), which is evaluating the efficacy, safety, pharmacokinetics, and pharmacodynamics of itolizumab for first-line treatment of aGvHD. The GvHD Hub has previously reported on interim findings from the EQUATE trial and published an expert interview on the promising results obtained when targeting CD6+ T cells in patients with aGvHD.
During the 48th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), Stephen Connelly1 presented the mechanism of action of itolizumab and pharmacokinetic data, while Corey Cutler2 presented the updated interim results from the EQUATE trial. Here, we summarize the key findings from these two presentations.
Mechanism of action
CD6-ALCAM pathway1,2
CD6 is a co-stimulatory receptor, predominantly expressed on T cells. CD6 binds to ALCAMs present on antigen presenting cells, and epithelial and endothelial cells present on various tissues, including skin, gut, and lung. The CD6-ALCAM pathway modulates T-cell activation, proliferation, and trafficking, and thus plays an integral part in inflammation and autoimmunity. Itolizumab binds to CD6 (at Domain-1) and causes shedding of the receptor. T cells with lower levels of CD6 exhibit reduced sensitivity to stimulation, including decreased activation, proliferation, and cytokine production (Figure 1).
Figure 1. Mechanism of action of itolizumab*
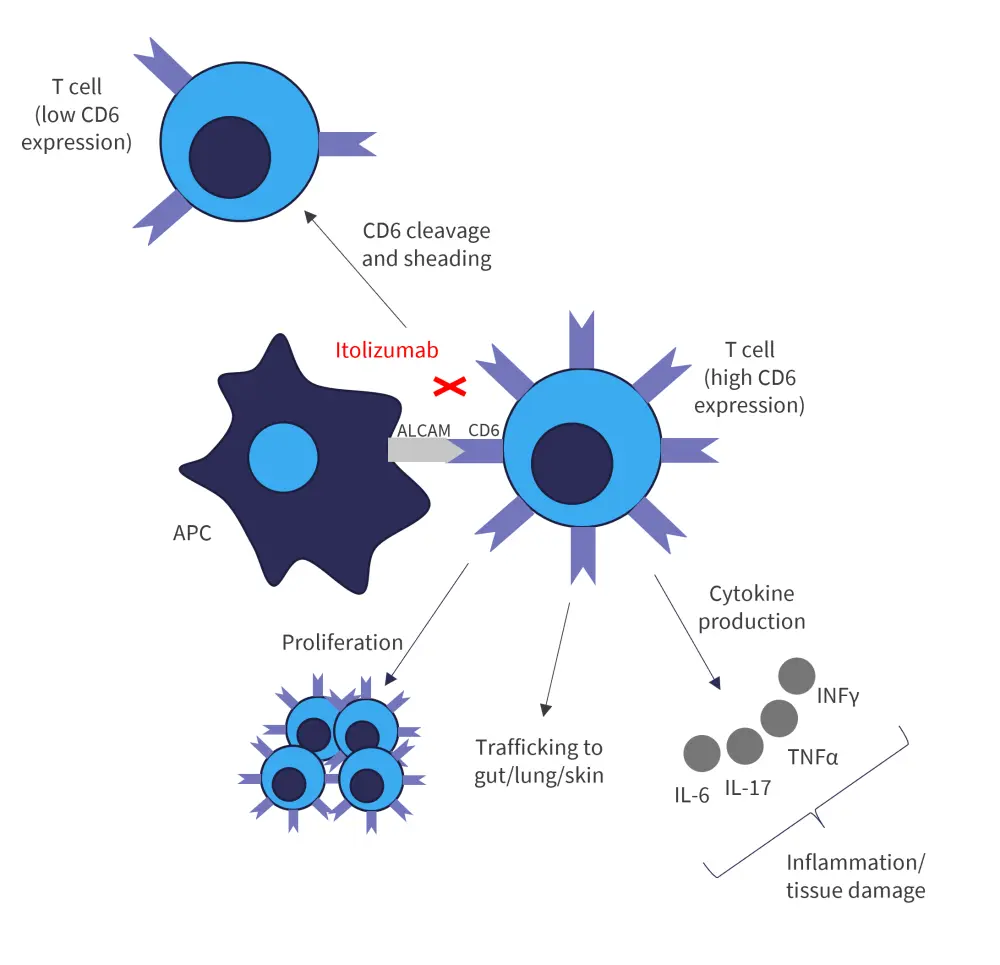
ALCAM, activated leukocyte cell adhesion molecule; APC, antigen presenting cell; CD6, cluster of differentiation-6; IFN-γ, interferon-γ; IL, interleukin; TNFα, tumor necrosis factor-α.
*Adapted from Connelly, et al.1 and Cutler, et al.2
EQUATE study design1,2
EQUATE is an ongoing phase Ib/II, open-label, dose-escalation, multi-center trial, evaluating the efficacy and safety profile of itolizumab in patients aged ≥18 years with Grade III–IV aGvHD, who have received their first allo-HSCT. Patients received itolizumab intravenously biweekly at doses 0.4 mg/kg, 0.8 mg/kg, and 1.6 mg/kg, and were followed up through Day 337 (Figure 2).
Figure 2. Treatment schema*†
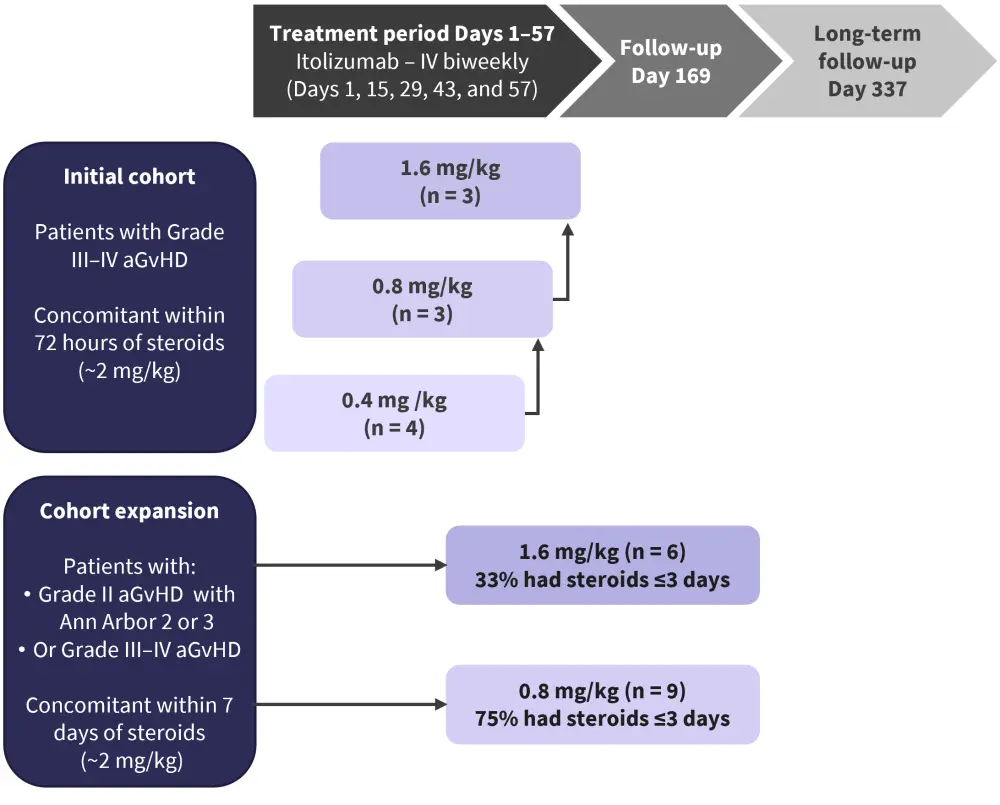
aGvHD, acute graft-versus-host disease; IV, intravenously.
*Adapted from Connelly, et al.1 and Cutler, et al.2
†Enrollment continued at 0.8 mg/kg with ≤3 days of prior steroids; no further dose escalation >1.6 mg/kg was conducted.
The primary objectives included assessment of the safety and tolerability of itolizumab, as well as determination of the optimal does levels.
The secondary objectives included characterization of the pharmacokinetics (PK) and pharmacodynamics (PD) of itolizumab and assessment of its clinical activity (aGvHD overall response rate [ORR], non-relapse mortality, chronic GvHD response, and durability)
Baseline characteristics2
All results presented are interim data for patients enrolled through 13 October 2021 and are subject to change.
A total of 25 patients were included; the median age was 59 years, and 64% and 32% of patients had Grade III and IV aGvHD, respectively (Table 1).
Table 1. Baseline patient characteristics*
|
aGvHD, acute graft-versus-host disease; AML, acute myeloid leukemia ; LGI, lower gastrointestinal involvement; MDS, myelodysplastic syndrome; UGI, upper gastrointestinal involvement. |
||||
|
Characteristic, % (unless otherwise stated) |
Itolizumab |
Itolizumab |
Itolizumab |
Total |
|---|---|---|---|---|
|
Mean age, years |
44 |
60 |
55 |
55 |
|
Male |
100 |
58 |
56 |
64 |
|
White |
100 |
75 |
89 |
84 |
|
Primary disease |
|
|
|
|
|
AML |
50 |
50 |
22 |
40 |
|
MDS |
0 |
25 |
22 |
20 |
|
Other |
50 |
25 |
56 |
40 |
|
Graft source |
|
|
|
|
|
Peripheral blood |
100 |
83 |
100 |
92 |
|
Bone marrow |
0 |
17 |
0 |
8 |
|
aGvHD grade† |
|
|
|
|
|
II |
0 |
8 |
0 |
4 |
|
III |
75 |
58 |
67 |
64 |
|
IV |
25 |
33 |
33 |
32 |
|
Organ involvement |
|
|
|
|
|
Skin |
25 |
33 |
44 |
36 |
|
Liver |
25 |
0 |
11 |
8 |
|
UGI |
25 |
67 |
22 |
44 |
|
LGI |
100 |
83 |
78 |
84 |
|
Minnesota high risk score‡ |
100 |
67 |
78 |
76 |
|
Ann Arbor Score 2 or 3 |
100 |
100 |
89 |
96 |
Results
Pharmacokinetics and pharmacodynamics1
An increase in itolizumab serum concentration levels was observed after the first dose and was proportional to the size of the dose.
The surface CD6 level on T cells following the first dose of itolizumab was reduced significantly (p < 0.001) and remained low throughout the treatment period. Of note, 0.4 mg/kg itolizumab was a suboptimal dose due to an increase in CD6 level trending towards baseline by Day 15. Itolizumab increased the ratio of regulatory T cells/effector T cells (compared with baseline) by Day 15 in the higher does cohorts (0.8 and 1.6 mg/kg) only, highlighting the potential for enhanced immune response. A decrease in protein death-1, CD25 (interleukin-2R), and CD95 was also observed over the dosing period. Patients who achieved a complete response (CR) on Day 15 showed higher trough concentrations of itolizumab in serum.
Efficacy2
Itolizumab demonstrated early responses at Day 15 in terms of CR rate and ORR (50% and 71%, respectively) and were maintained at Day 29 (52% and 64%, respectively). Patients receiving itolizumab within 72 hours of corticosteroids showed a higher CR rate and ORR of 61% and 67%, respectively, at Day 29 (Table 2). In addition, itolizumab also showed a durable response rate of 79% at Day 169 across all doses and the response rate was maintained in 55% of responders at Day 337. At 12 months, progression-free survival and overall survival in Day 29-responders was ~50% and >75%, respectively. The median steroid reduction for responders was 73% and 96% at Day 29 and Day 169, respectively.
Table 2. CR and ORR*
|
CR, complete response; ORR, overall response rate. §Response data were not available for one patient. |
|||||||||
|
Itolizumab dose |
Response at Day 15 |
Response at Day 29 |
Naïve response at Day 29† |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
n |
CR, % |
ORR‡, % |
n |
CR, % |
ORR‡, % |
n |
CR, % |
ORR‡, % |
|
|
0.4 mg/kg (n = 4) |
4 |
50 |
75 |
4 |
50 |
50 |
4 |
50 |
50 |
|
0.8 mg/kg (n = 12) |
11§ |
55 |
64 |
12 |
58 |
58 |
9 |
67 |
67 |
|
1.6 mg/kg (n = 9) |
9 |
44 |
78 |
9 |
44 |
78 |
5 |
60 |
80 |
|
Total |
24§ |
50 |
71 |
25 |
52 |
64 |
18 |
61 |
67 |
Safety2
In total, 64% of patients presented with a serious adverse event (SAE), including 40% reporting an infection SEA and 8% reporting a treatment-related SAE. Six patients discontinued due to AEs, including three due to infections. There was one dose-limiting toxicity at the 1.6 mg/kg dose. A total of 11 patients died, including eight due to AEs leading to death. However, there were no treatment-related deaths (Table 3).
Table 3. AEs and SAEs*
|
AE, adverse event; CTCAE, common terminology criteria for adverse events, SAE, serious adverse event. |
||||
|
AEs and SAEs, % |
Itolizumab |
Itolizumab |
Itolizumab |
Total |
|---|---|---|---|---|
|
Any AE |
100 |
100 |
100 |
100 |
|
Infusion reaction AE |
25 |
17 |
0 |
12 |
|
Infection AEs |
75 |
50 |
78 |
64 |
|
CTCAE Grade ≥3 AE |
50 |
75 |
100 |
80 |
|
Treatment discontinuation |
|
|
|
|
|
Any AEs |
0 |
17 |
44 |
24 |
|
Infection AEs |
0 |
8 |
22 |
12 |
|
SAE |
50 |
50 |
89 |
64 |
|
Infection SAE |
25 |
25 |
67 |
40 |
|
Treatment-related SAE |
0 |
8 |
11 |
8 |
|
Dose-limiting toxicity AE† |
0 |
0 |
11 |
4 |
|
Deaths |
25 |
42 |
56 |
44 |
|
AE leading to death |
0 |
42 |
33 |
32 |
|
Treatment-related mortality |
0 |
0 |
0 |
0 |
Conclusion
The updated interim findings from the EQUATE trial demonstrate that itolizumab is responsible for a rapid and durable decrease in cell surface CD6, including reduction in the ratio of activated effector T cells to regulatory T cells. A higher concentration of itolizumab in serum highlights the importance of achieving these concentrations earlier in the treatment period to maximize the efficacy. Itolizumab offers a deep and durable response, including a favorable risk profile. In a patient population with severe aGvHD, itolizumab showed tolerability across all doses. Based on these interim findings, a pivotal phase III study has been initiated to investigate the efficacy and safety of itolizumab versus placebo as first-line-therapy in patients with Grade III–IV aGvHD (NCT05263999).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

