All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Phase II trial of urinary-derived human chorionic gonadotropin/epidermal growth factor for the treatment of aGvHD
Featured:
Alternative treatments beyond corticosteroids are needed for the treatment of acute graft-versus-host disease (aGvHD). To improve long-term survival, treatments are needed that do not focus on immunosuppression alone. Favoring inflammation resolution, improving tissue repair, and working towards immune tolerance may produce better long-term results with respect to survival.
One of the agents being investigated with respect to steroid-sparing therapies is urinary-derived human chorionic gonadotropin/epidermal growth factor (uhCG/EGF), which was granted orphan drug designation in 2020 based on the results of a phase I/II trial (NCT02525029).
During the 63rd American Society of Hematology Annual Meeting & Exposition (ASH), Shernan Holtan presented a talk on the phase II portion of this trial of uhCG/EGF for aGvHD,1 which we are pleased to summarize below.
The GvHD Hub was also able to speak to Shernan Holtan during ASH; in this video she discusses where uhCG/EGF could fit into current aGvHD treatment pathways.
How could uhCG/EGF fit into current aGvHD treatment pathways?
Study design
The inclusion criteria for the trial comprised:
- Life-threatening aGvHD
- Aged between 0–76 years
- Creatinine levels <2.5× upper limit of normal
- A left ventricular ejection fraction ≥35%
Life-threatening aGvHD for patients receiving first-line therapy was defined as high-risk aGvHD according to the Minnesota GvHD risk score calculator.2 For second-line patients, the definition included having no response to first-line treatment or experiencing a GvHD flare up. Patients receiving first-line therapy or second-line therapy were divided into the Minnesota high-risk (MHR) or second-line treatment (2LT) groups, respectively.
Patients were excluded from the trial if they had any of the following:
- An active and progressive malignancy
- A malignancy with a history of hormone responsiveness
- An uncontrolled infection
- Thromboembolic disease within 3 months
- Supplemental hormone therapy that they were unwilling or unable to stop
The primary endpoint was outcome at Day 28. Secondary outcomes looked at safety, survival, exploratory metabolomics analysis, and biomarkers.
The dosing schedule used in the trial is shown in Figure 1. Optional maintenance treatment was available to all responders twice a week for 5 weeks.
Figure 1. Dosing schedule*
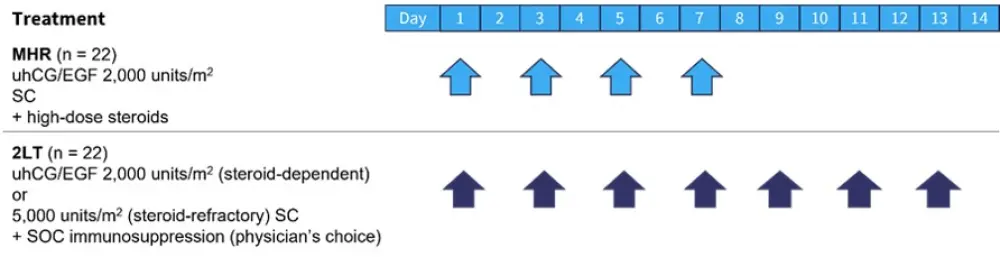
2LT, second-line treatment; EGF, epidermal growth factor; MHR, Minnesota high-risk; SC, subcutaneously; SOC, standard of care; uhCG, urinary-derived human chorionic gonadotrophin.
*Data from Holtan.1
Results
Patient characteristics
Patient characteristics, including age and sex, were comparable between the two groups, as shown in Table 1. Notably, the median Karnofsky performance status was slightly higher in the MHR group, and more patients in the 2LT group were given reduced intensity conditioning.
In the MHR group, a majority of patients had Stage III−IV lower gastrointestinal aGvHD. At enrollment the MHR group were mostly classified as Grade 3−4 aGvHD (Table 1).
Table 1. Baseline characteristics*
|
2LT, second-line treatment; aGvHD, acute graft-versus-host disease; ATG, anti-thymocyte globulin; KPS, Karnofsky performance score; MHR, Minnesota high-risk. |
||
|
Characteristic |
MHR |
2LT |
|---|---|---|
|
Median age (range), years |
61 (22−72) |
62 (2−69) |
|
Median KPS (range) |
60 (30−90) |
50 (20−100) |
|
Male, % |
73 |
77 |
|
Graft source, % |
|
|
|
Marrow |
23 |
36 |
|
Peripheral blood stem cells |
36 |
41 |
|
Umbilical cord blood |
41 |
23 |
|
Conditioning, % |
|
|
|
Myeloablative |
45 |
23 |
|
Reduced intensity |
55 |
77 |
|
Median post-transplant day of enrollment (interquartile range) |
57 (34−118) |
123 (76−209) |
|
aGvHD organ stage at enrollment, % |
|
|
|
Skin |
|
|
|
0 |
59 |
55 |
|
I−II |
19 |
19 |
|
III−IV |
23 |
27 |
|
Lower gastrointestinal |
|
|
|
0 |
5 |
36 |
|
I−II |
28 |
28 |
|
III−IV |
68 |
36 |
|
Liver |
|
|
|
0 |
86 |
82 |
|
I−II |
5 |
19 |
|
III−IV |
9 |
0 |
|
aGvHD clinical grade at enrollment, % |
|
|
|
2 |
9 |
41 |
|
3 |
77 |
23 |
|
4 |
14 |
36 |
|
Median baseline albumin (range), g/dL |
2.7 (1.9−3.4) |
2.5 (0.9−4.0) |
|
Concomitant GvHD therapy, n |
|
|
|
Steroids |
22 |
8 |
|
ATG |
0 |
4 |
|
Etanercept |
0 |
1 |
|
Ruxolitinib |
0 |
5 |
|
Sirolimus |
0 |
4 |
Efficacy
Looking at the primary outcome of response at Day 28 for the entire cohort, 57% patients achieved a complete response (CR) and 11% showed a partial response (PR). Analysis of the two groups of interest showed that 64% achieved CR in the MHR group compared with 50% in the 2LT group (Figure 2).
Figure 2. Response at Day 28*
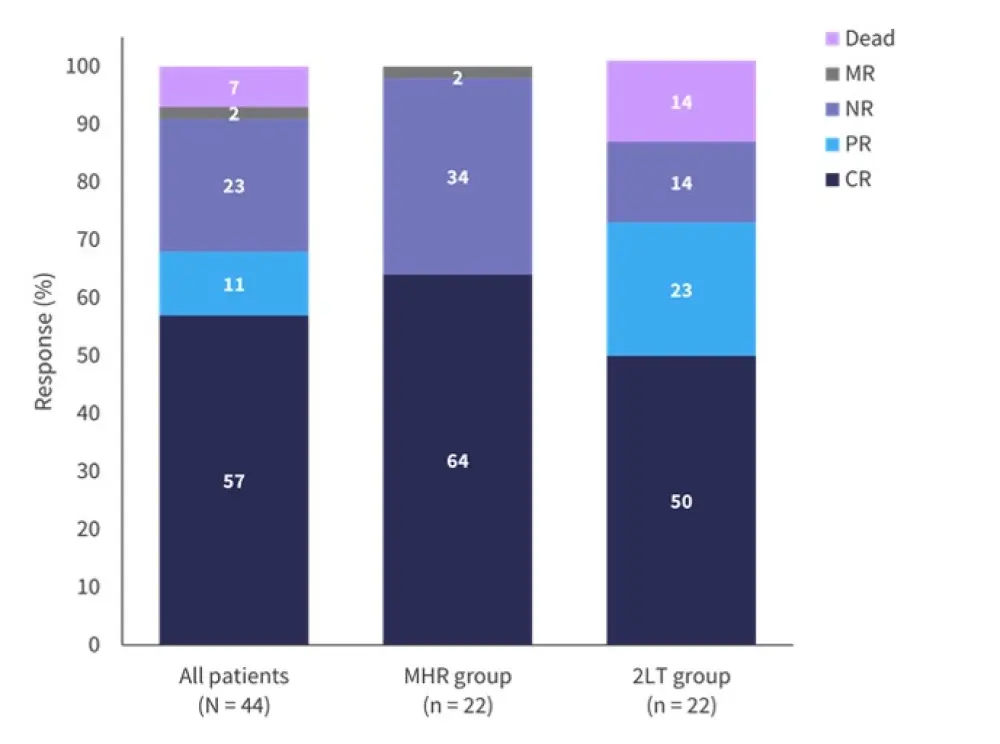
2LT, second-line treatment; CR, complete response; MHR, Minnesota high-risk; MR, minimal response; NR, no response; PR, partial response.
*Adapted from Holtan.1
Median overall survival (OS) for the whole cohort was 1.2 years, and was similar between the MHR and 2LT groups (p = 0.52). The 2-year OS was 67% (52−86%) for responders at Day 28 compared with 12% (2−72%) for non-responders (p < 0.01).
Non-relapse mortality was similar for both groups at 2-years (p = 0.66). When patients were separated according to response at Day 28, a trend towards significance was seen for responders compared with non-responders (p = 0.09).
Safety
The most common adverse events (AEs) were injection site reactions, infections, and those affecting the gastrointestinal, nervous, and vascular systems. Most AEs were Grade 1−3. Only one case of dose-limiting toxicity was recorded, which resulted in an incidental cerebral venous sinus thrombosis that was treated successfully.
Overall, 52% of patients included on the trial died, with a median follow-up of 17 months. The breakdown for causes of death is shown in Figure 3; relapse and GvHD were the largest contributors to mortality.
Figure 3. Causes of death*
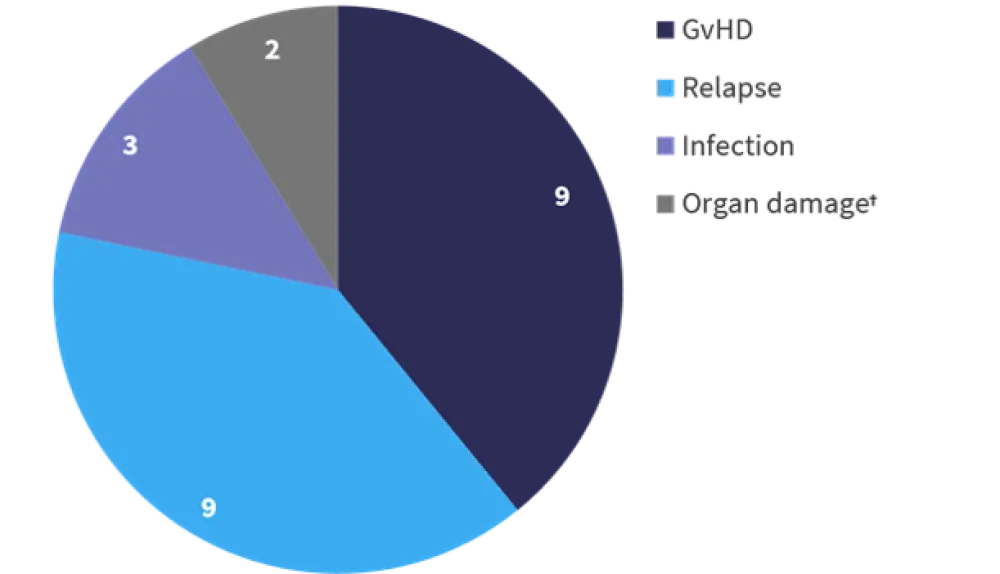
GvHD, graft-versus-host disease.
*Adapted from Holtan.1
†Organ damage unrelated to GvHD.
Exploratory metabolomics analysis
Linoleic acid concentration was investigated and increased levels were found to be associated with a response to therapy. Whereas lactic acid showed the opposite, with increased levels being associated with no response. The high levels of lactic acid were thought to indicate that these patients experienced tissue hypoxia and were more critically ill.
Conclusion
Results from using uhCG/EGF with systemic treatment produced a CR/PR rate of 68% for the whole cohort at Day 28. In addition, uhCG/EGF plus systemic therapy improved OS in a group that traditionally has a very low life expectancy. This trial highlights the possible association of metabolomic profiling and response and demonstrates that GvHD and relapse remains an unmet need.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


 Shernan Holtan
Shernan Holtan