All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Outcomes, biomarkers, and risk factors in de novo late aGvHD
Graft-versus-host disease (GvHD) causes significant morbidity and mortality following allogeneic hematopoietic stem cell transplantation (HSCT). Before 2005, GvHD was classed as acute if it occurred before Day 100 and chronic if it occurred after Day 100 post-HSCT. However, the 2005 National Institutes of Health Consensus Conference proposed a new classification based solely on clinical manifestations, rather than time of onset. De novo acute GvHD (aGVHD) is defined as GvHD that presents beyond Day 100 post-HST but does not clinically manifest as chronic GvHD (cGvHD); it can often be under-recognized due to limited data on its characteristics and risk factors.
This retrospective analysis compared patients from 24 transplant centers across North America, Europe, and Asia. Patients with aGvHD were included, they were spilt into classic aGvHD and late aGvHD based on whether GvHD occurred before or after Day 100 post-HSCT.
Study design
A total of 3,542 patients who received HSCT at a Mount Sinai Acute GvHD International Consortium (MAGIC) center between January 2014 and August 2021 were included for analysis. Eligible patients were aged ≥18 years and had received their first HCT from a human leukocyte antigen (HLA)-matched related donor, HLA-matched unrelated donor, HLA-mismatched unrelated donor, or haploidentical donor.
Key factors examined in this retrospective analysis included incidence, clinical presentation, risk factors, outcomes, and value of GvHD biomarkers. Serum samples were collected at the time of systemic treatment and at Week 1 of treatment. The MAGIC algorithm probability (MAP) was calculated as a value between 0.001 and 0.999.
Results
Of surviving patients, 52.4% were diagnosed with aGvHD of any grade. Classic and late aGvHD was identified in 45.2% and 7.2% of patients, respectively (Figure 1). The median follow-up for survivors was 722 days post-HCT.
Figure 1. Patient inclusion*
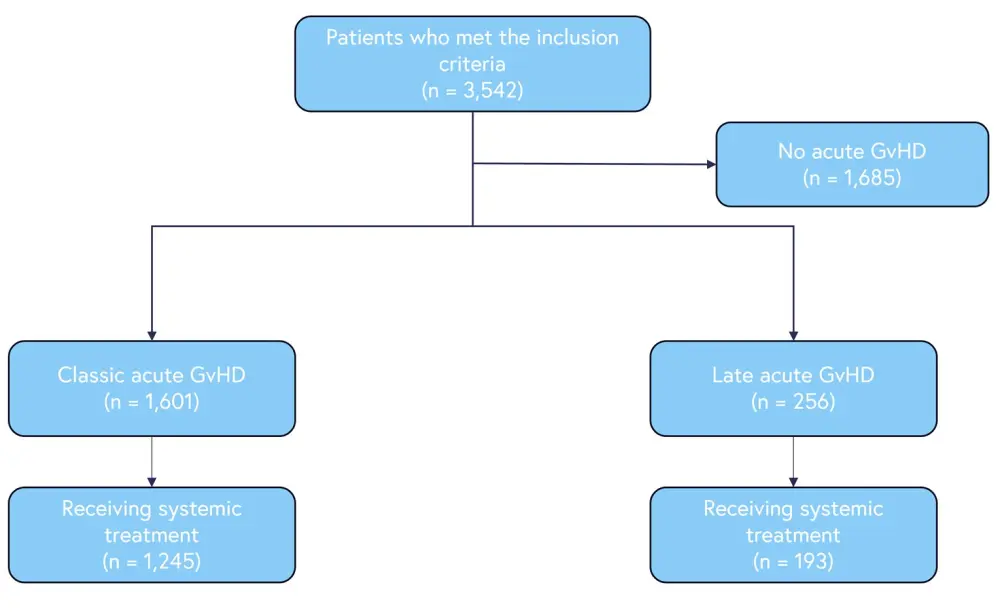
GvHD, graft-versus-host disease.
*Adapted from Akahoshi, et al.1
Overall, 77.8% of patients had classic aGvHD and required systemic treatment, while 35.2% required systemic treatment for late aGVHD. Baseline patient characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
GvHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplant; HLA, human leukocyte antigen; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor. |
||
|
Characteristic, n (%) (unless otherwise stated) |
Classic (n = 1,245) |
Late (n = 193) |
|---|---|---|
|
Median age at HSCT (range), years |
68 (18–79) |
61 (19–78) |
|
Recipient age |
|
|
|
<55 |
392 (31.5) |
33 (17.1) |
|
≥55 |
853 (68.5) |
160 (82.9) |
|
Sex mismatch |
|
|
|
Other |
1,057 (84.9) |
145 (75.1) |
|
Female to male |
188 (15.1) |
48 (24.9) |
|
Disease risk |
|
|
|
Standard |
991 (79.6) |
165 (85.5) |
|
High |
254 (20.4) |
28 (14.5) |
|
Donor type |
|
|
|
HLA-MRD |
256 (20.6) |
86 (44.6) |
|
HLA-MUD |
708 (56.9) |
88 (45.6) |
|
HLA-MMUD |
131 (10.5) |
8 (4.1) |
|
Haploidentical donor |
150 (12.0) |
11 (5.7) |
|
Donor source |
|
|
|
Bone marrow |
211 (16.9) |
19 (9.8) |
|
Peripheral blood |
1034 (83.1) |
174 (90.2) |
|
GvHD grades |
|
|
|
I |
401 (32.2) |
34 (17.6) |
|
II |
624 (50.1) |
89 (46.1) |
|
III |
172 (13.8) |
59 (30.6) |
|
IV |
48 (3.9) |
11 (5.7) |
Outcomes
Overall GvHD grades were significantly higher in patients with late aGvHD compared to classic aGvHD. At Day 28, the overall response rate was significantly higher in patients with classic aGvHD compared to patients with late aGvHD, at 72% and 55.4%, respectively; classic aGvHD was also associated with a decreased risk of disease relapse. At 6 months, there was no significant difference in non-relapse mortality between the groups.
Biomarkers
Serum samples were available at baseline in 83.6% and 46.1% of patients with classic aGvHD and late aGvHD, respectively. Of these, more patients with late aGvHD had high-risk MAGIC algorithm probability (MAP) biomarkers (29.2% vs 16.2%). After 1 week of treatment, a greater proportion of patients with late aGvHD had high MAP scores of ≥0.29 compared with patients with classic aGvHD (33.7% vs 16%). Higher MAP scores were associated with a significantly greater risk of non-relapse mortality in both patient groups.
Risk factors for late aGvHD
Multivariate analysis showed that no relapse by Day 100, no history of classic aGvHD, older recipient age, female donor to male recipient sex mismatch, and the use of reduced intensity conditioning was significantly associated with increased risk of late aGvHD. Conversely, using posttransplant cyclophosphamide-based prophylaxis significantly reduced the risk of late aGvHD.
Conclusion
This retrospective analysis suggests that the severity of late aGvHD may be greater than classical aGvHD at onset, as outlined by biomarkers and clinical signs. In addition, overall response was greater in patients with classical aGvHD compared with late aGvHD.
This study is limited by a relatively low number of patients included with late aGvHD. In addition, treatment decisions outside of clinical trials are not consistent due to the practices of individual centers and investigators. However, this study shows that late aGvHD should be considered when testing novel treatments and can be treated with the same intensity as classical aGvHD.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

