All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Impaired development of regulatory T cells (Tregs) following hematopoietic stem cell transplant (HSCT) has been associated with enhanced graft-versus-host disease (GvHD), causing significant rates of morbidity and mortality. At the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, Everett Meyer spoke about Orca-T, an engineered Treg donor product, as an agent for treating acute GvHD (aGvHD).1 Orca-T was awarded regenerative medicine advanced therapy and orphan drug designation by the U.S. Food and Drug Administration (FDA) in 2019. See our interview with Meyer here and another interview with Barbara Fazekas de St Groth on why Treg reconstitution is important to prevent GvHD.
Orca-T is a CD34-selected Treg-engineered graft that has shown promising initial results but requires further testing. Compared with traditional HSCT, the donor cells in Orca-T are processed and the Tregs, hematopoietic stem cells, and progenitor cells are separated out from the conventional T cells and infused back into the patient according to the schema in Figure 1.
Figure 1. Protocol schema2
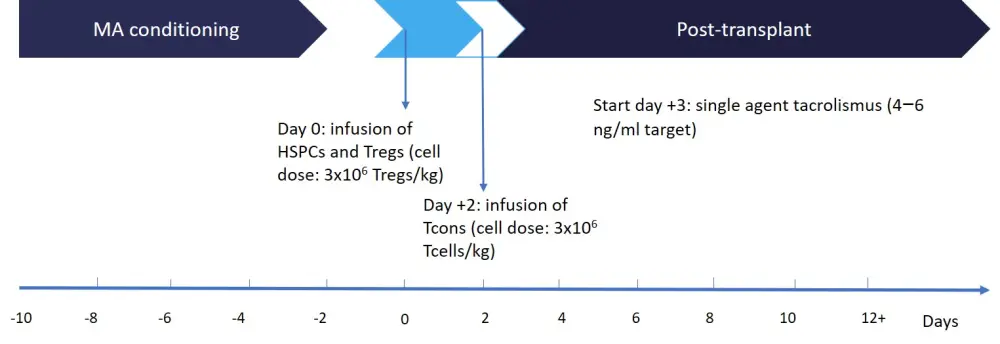
HSPCs, hematopoietic stem and progenitor cells; MA, myeloablative; Tcon, conventional T cell; Treg, regulatory T cell.
Study design
This analysis includes data from phase Ib (n = 11; NCT04013685) and phase I/II (n = 40; NCT01660607) trials.
Purification of Tregs was performed by immunomagnetic selection of CD25+ cells of granulocyte colony-stimulating factor-mobilized peripheral blood cells followed by flow cytometric cell sorting of CD4+CD127lo cells. The infused target dose was 2–3 million Tregs/kg with a 1:1 ratio of conventional T cells and Tregs.
The primary objective of the studies was the rate of GvHD relapse-free survival (GRFS) at 1 year. Secondary objectives were dose limiting toxicity, overall survival, incidence and severity of chronic GvHD, and incidence of infections.
Results were compared with a historical standard of care (SoC) cohort treated at Stanford University Medical Center (N = 138) with both matched related (n = 79) and unrelated (n = 59) allo-HSCT consisting of unmanipulated peripheral blood stem cell products. Patients were treated with methotrexate plus tacrolimus for GvHD prophylaxis.
Eligibility criteria:
- Patients with high-risk, measurable residual disease-positive active disease (leukemia, lymphoma, myelodysplastic syndromes, or myeloproliferative neoplasms) and candidates for allo-HSCT with a matched related or unrelated donor.
- Patients aged < 65 years with a Karnofsky Performance Status score > 70 and adequate organ function.
Table 1. Baseline patient characteristics1
|
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BCL, B-cell lymphoma; CML, chronic myeloid leukemia; HLA, human leukocyte antigen; MDS, myelodysplastic syndromes; MF, myelofibrosis; SoC, standard of care; URD, unrelated donor. |
||
|
|
Orca-T (N = 50)* |
SoC control cohort (N = 144) |
|---|---|---|
|
Median age, years (range) |
47 (20–65) |
48 (20–64) |
|
Male, % |
52 |
49 |
|
Ethnicity, % |
|
|
|
White |
60 |
44 |
|
African American |
2 |
2 |
|
Asian |
14 |
19 |
|
Unspecified |
26 |
30 |
|
Primary disease, % |
|
|
|
AML |
42 |
53 |
|
ALL |
28 |
26 |
|
CML |
4 |
7 |
|
BCL |
2 |
6 |
|
MDS/MF |
16 |
22 |
|
Other† |
8 |
2 |
|
Graft source, % |
|
|
|
HLA-matched siblings |
62 |
56 |
|
URD |
38 |
44 |
|
Active leukemia at time of transplant, % |
23 |
21 |
|
Median follow-up, days (range) |
223 (30−1,561) |
886 (55−1,783) |
Key points
- The study was carried out in > 12 clinical sites across the US.
- The products were sourced from matched related and matched unrelated donors and produced with a vein-to-vein time of < 72 hours without manufacturing nor logistic failures.
- Orca-T was found to result in significantly quicker engraftment and reduced hospital stays for patients compared with the current SoC.
- Furthermore, Orca-T significantly reduced the incidence of severe acute and chronic GvHD, and treatment-related mortality, while it improved relapse-free survival (Table 2).
Table 2. Engraftment length and hospital stay duration1
|
aGvHD, acute graft-versus-host disease; cGvHD, chronic graft-versus-host disease; GRFS, graft-versus-host disease relapse-free survival; SoC, standard of care. |
|||
|
|
Orca-T |
SoC |
p value |
|---|---|---|---|
|
Median time to neutrophil engraftment, days |
12 |
14 |
< 0.0001 |
|
Median time to platelet engraftment, days |
11 |
17 |
< 0.0001 |
|
Median time from Day 0 hospital discharge, days |
15 |
17 |
0.01 |
|
aGvHD Grade ≥ 2 at Day 100, % |
10 |
30 |
0.005 |
|
cGvHD at 1 year, % |
3 |
46 |
0.0002 |
|
GRFS at 1 year, % |
75 |
31 |
0.001 |
|
Treatment-related mortality at 1 year, % |
0 |
11 |
0.04 |
Table 3 shows a doubling of the number of patients achieving a complete response with measurable residual disease-negativity at 90 days after treatment with Orca-T and this was maintained for 180 days. The response lasted for a year in 11 patients. This included four patients with active/refractory disease/partial response before Orca-T administration.
Table 3. Duration of response and variation over time1
|
CR, complete response; MRD, measurable residual disease; PR, partial response. |
||||
|
|
CR, MRD‑negative |
CR, MRD‑positive |
Active/refractory disease/PR |
Deaths |
|---|---|---|---|---|
|
Pre-Orca-T, n |
12 |
6 |
9 |
0 |
|
Day 90, n |
24 |
1 |
2 |
0 |
|
Day 180, n |
24 |
1 |
2 |
0 |
|
Day 356, n |
11 |
1 |
1 |
2 |
Conclusion
Orca-T was able to effectively reduce the incidence of acute and chronic GvHD compared with the current SoC. No treatment-related mortality was recorded in patients treated with Orca-T, and relapse-free survival was more than doubled compared with the SoC cohort. While further testing in larger clinical trials is required, these initial results are promising. Orca-T is currently being assessed in an ongoing multicenter trial.
Expert Opinion
 Everett H. Meyer
Everett H. MeyerReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

