All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Novel drugs for treating cGvHD: Phase I results of axatilimab for heavily pretreated patients
Second line or further lines of therapy represent an unmet need in treating patients with chronic graft-versus-host disease (cGvHD) due to disease progression or the lack of response. Axatilimab is a novel, humanized, immunoglobulin G4 (IgG4) antibody inhibiting colony-stimulating factor 1 receptor (CSF-1R) agent, which is currently under development for the treatment of patients with refractory cGvHD following ≥2 lines of systemic therapy. CSF-1/CSF-1R pathway has been shown to associated with the growth and infiltration of donor-derived macrophages leading to cGvHD.
During the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, Mukta Arora presented the preliminary phase I results1, and this article summarizes the key points.
Study design
The study population included patients ≥6 years of age, with active cGvHD following ≥ 2 lines of prior therapies, and who had Karnofsky performance score ≥60%. Corticosteroids and calcineurin inhibitors were allowed.
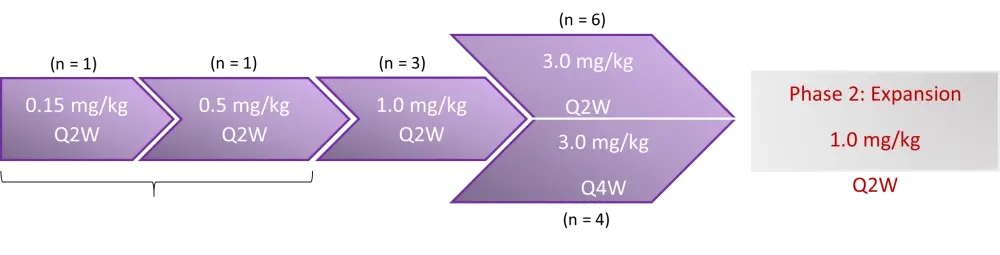
Endpoints were safety, tolerability, overall response rate (ORR), and recommended Phase 2 dose (RP2D). Dose escalation design is depicted in Figure 1.
Figure 1. Study design (adapted from Arora et al., 2020)1
Baseline characteristics
Total number of patients was 15 with a median age of 60 years (range, 29–73 years). Almost half (47%) of patients underwent myeloablative transplant. Peripheral blood stem cells (PBSCs) were used as graft source in 93% of patients. The median time from cGvHD to Cycle 1 Day 1 (C1D1) was 42 months (range, 9.6–187.2 months). Median number of organs involved was 4 (range, 1–9), and median number of prior treatments was 4 (range, 2–9). Prior therapies included ibrutinib, ruxolitinib, and KD025.
Results
Treatment-emergent adverse events (TEAEs) that were considered related to axatilimab, occurred in all patients among different dose ranges. Grade 3–4 events occurring in ≥ 2 patients comprised elevated creatine kinase levels (n = 3), aspartate aminotransferase increase (n = 2), and pneumonia (n = 2). Infectious events were observed in six patients including pneumonia, conjunctivitis, norovirus gastroenteritis, influenza, lung infection, Pseudomonas infection in the foot, and upper respiratory infection. Reactivation of cytomegalovirus was not observed. The results of safety analysis are shown in Table 1.
Table 1. Safety outcomes2
|
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; Q2W, every 2 weeks, Q4W, every 4 weeks; TEAE, treatment-emergent AE. *0.15 mg/kg Q2W. †0.5 mg/kg Q2W. ‡All grades regardless of causality. |
||||
|
Outcome, n (%) |
< 1 mg/kg Q2W n = 2 |
1 mg/kg Q2W n = 3 |
3 mg/kg Q2W n = 6 |
3 mg/kg Q4W n = 4 |
|---|---|---|---|---|
|
Grade 3–4 TEAE |
2 (100) |
2 (67) |
4 (67) |
2 (50) |
|
Related Grade 3–4 TEAE |
1 (50)* |
1 (33) |
3 (50) |
2 (50) |
|
Treatment discontinuation Progression AE Physician decision Death Other |
2 (100) 1 (100)* 0 1 (100)† 0 0 |
2 (67) 1 (33) 0 0 1 (33) 0 |
3 (50) 0 1 (17) 1 (17) 0 1 (17) |
1 (25) 0 0 1 (25) 0 0 |
|
TEAEs occurring ≥ 5 patients‡ Elevated AST Elevated CPK Elevated LDH Elevated amylase Fatigue Elevated lipase Elevated ALT Elevated creatinine Nausea Pyrexia |
1 (50)* 0 1 (50)† 1 (50)* 1 (50)† 0 1 (50)* 0 2 (100) 0 |
1 (33) 1 (33) 2 (67) 1 (33) 0 1 (33) 0 1 (33) 0 1 (33) |
4 (67) 5 (83) 4 (67) 4 (67) 3 (50) 3 (50) 3 (50) 2 (33) 3 (50) 4 (67) |
3 (75) 3 (75) 2 (50) 0 2 (50) 2 (50) 1 (25) 2 (50) 0 0 |
Efficacy
Responses were achieved at all dose levels with deep and durable responses observed in different organ systems:
- CR in esophagus, and lower gastrointestinal tract (100%)
- CR in mouth (56%)
- PR in joints and fascia (55%), lungs (40%), and skin (40%)
- CR and PR in eyes (33%)
The median time to response was 1.9 months (range 1–11 months), and ORR was 57%. Lee symptom scores were also improved in most patients.
Conclusion
This patient population represents a heavily pretreated, recurrent/refractory active cGvHD cohort. In this study, axatilimab has shown good tolerability with low infection rates, and clinical activity was shown with responses in different organ systems, and following treatment with ibrutinib, ruxolitinib, and KD025; however, the limitation of small sample size was acknowledged. A phase II study (AGAVE-201) is planned to further investigate the efficacy and safety of axatilimab with three different doses.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

