All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Management of steroid-refractory chronic graft-versus-host disease
Chronic graft-versus-host disease (cGvHD) occurs in ≥30% of patients who survive ≥100 days following allogenic hematopoietic stem cell transplantation, and the complex and multifactorial pathogenesis of cGvHD—a disease with significant patient burden and effect on quality of life—can make diagnosis and treatment difficult. Mild cGvHD is generally managed with topical steroids, while moderate to severe disease requires systemic corticosteroids with or without a calcineurin inhibitor; however, more than half of patients become either steroid-resistant or steroid-dependent.1
Steroid-refractory cGvHD (SR-cGvHD) is defined by a lack of response to steroids, and while there are a number of alternative treatment options available, there is no consensus on an approach to treatment of SR-cGvHD and therefore no standard second-line treatments currently exist. Daniel Wolff and colleagues reviewed the literature and provided a review of current treatment options as well as a series of theoretical case studies, which we summarize here.1
Therapeutic options
Therapeutic interventions have been evaluated in clinical trials, though these results are difficult to interpret due to variations in patient populations and study design. Some of the currently available treatment options available for patients with SR-cGvHD are presented in Table 1. The Bruton’s tyrosine kinase inhibitor ibrutinib is the only therapy for SR-cGvHD that is approved by the U.S. Food and Drug Administration (FDA).
Table 1. Therapy options for SR-cGvHD*
|
AST, aspartate aminotransferase; cGvHD, chronic graft-versus-host disease; CPK, creatine phosphokinase; FFS, failure-free survival; GGT, gamma-glutamyltransferase; SR-cGvHD, steroid-refractory cGvHD; URTI, upper respiratory tract infection. |
|||||
|
Therapy |
Recommendation |
Evidence† |
Overall survival‡ |
Toxicities |
Study type |
|---|---|---|---|---|---|
|
Ibrutinib |
2nd line |
III–1 |
71% at 2 years in cGvHD |
Pneumonia, impaired platelet function |
Phase 2a |
|
Extracorporeal photopheresis |
2nd line |
II |
53–78% at 1 year |
Vascular access complications |
Phase 2 randomized |
|
Mycophenolate mofetil |
2nd line |
III–1 |
67–96% at 1 year |
Viral reactivation, hypertension, pneumonia, posttransplantation lymphoproliferative disease |
Retrospective cohorts |
|
Rituximab |
2nd line |
II |
72% at 1 year; 76% at 2 years |
Infections, infusion-related symptoms, late neutropenia |
Phase 2b randomized |
|
Ruxolitinib |
2nd line |
II |
97% at 6 months |
Viral reactivation/infection, peripheral neuropathy, cytopenias, malignancy relapse |
Phase 3 randomized |
|
mTOR inhibitors |
2nd line |
III–1 |
— |
Thrombotic microangiopathy, renal insufficiency, proteinuria |
Phase 2a |
|
Imatinib |
2nd line |
II |
84% at 1.5 years |
Fluid retention, myelosuppression, anemia |
Phase 2b |
|
Methotrexate |
2nd line |
III–1 |
96% at 1 year; 90% at 1.5 years |
Hepatotoxicity, leukopenia, thrombocytopenia |
Retrospective cohorts |
|
Pentostatin |
>2nd line |
II |
78% at 1 year; 70% at 2 years |
Infections |
Phase 2a |
|
IL-2 therapy |
>2nd line |
III–1 |
Under investigation |
Injection site induration, infections |
Phase 2 |
|
Pomalidomide |
>2nd line |
III–1 |
In a phase 1/2 study, all responders were still alive after a median follow-up of 4.6 years |
Lymphopenia, neutropenia, infections, muscle cramps, fatigue; early use after transplant may increase risk for inflammatory flares |
Phase 2 |
|
Ixazomib |
>2nd line |
III–2 |
90% at 12 months |
— |
Phase 2 |
|
Low-dose total lymphoid irradiation |
>2nd line |
III–2 |
Median 13 months in responders vs 10 months in non-responders |
Thrombocytopenia, neutropenia |
Retrospective cohorts |
|
Mesenchymal stem cells |
>3rd line |
III–2 |
78% at 2 years |
None reported |
Phase 2 |
|
Thalidomide |
>3rd line |
II |
41% at 2 years in SR-cGvHD |
Birth defects, constipation, rash, fatigue, somnolence, neuropathy |
Phase 2 |
|
Alefacept |
>3rd line |
III–2 |
50% at 30 months |
No dose-limiting toxicities |
Phase 1 |
|
Abatacept |
>3rd line |
III–2 |
— |
No dose-limiting toxicities were identified |
Phase 1 |
|
Tocilizumab |
>3rd line |
III–2 |
82% with median follow-up of 22 months |
Infections, granulocytopenia, thrombocytopenia |
Retrospective cohorts |
|
Cyclophosphamide |
>3rd line |
III–2 |
— |
Short-term myelosuppression, neutropenia, fatigue, nausea |
Retrospective cohorts |
|
Baricitinib |
>3rd line |
III–2 |
FFS 74% at 1 year, 37% at 2 years |
Viral reactivation, neutropenia, hypophosphatemia, hypertriglyceridemia, URTI |
Phase 1/2 single arm |
|
Belumosudil§ |
Available in clinical trials only |
III–1 |
FFS 77% at 6 months |
Pneumonia, hypertension, hyperglycemia, increased GGT |
Phase 2 open label randomized |
|
Axatilimab |
Available in clinical trials only |
III–2 |
— |
Increased GGT, AST, and CPK, periorbital edema |
Phase 1/2 dose escalation and expansion |
Several factors should be considered when selecting treatment for patients with SR-cGvHD, including the patient’s disease history and comorbidities, as well as available published evidence and access to clinical trials; this is largely a patient-specific decision.
Case presentation: patient 1
The characteristics and initial treatment information for patient 1 are detailed in Figure 1.
Figure 1. Patient 1: characteristics and treatment information*
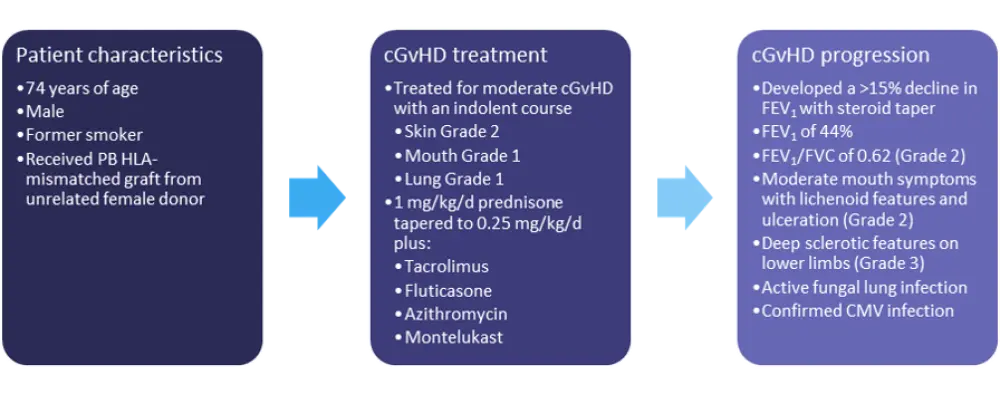
cGvHD, chronic graft-versus-host disease; CMV, cytomegalovirus; d, day; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; HLA, human leukocyte antigen; PB, peripheral blood.
*Adapted from Wolff, et al.1Treatment options
The best option for most patients with SR-cGvHD is enrollment in a clinical trial, though this patient would likely be ineligible due to his active fungal infection. The use of ruxolitinib, ibrutinib, or mycophenolate mofetil (MMF) should also be avoided due to the potential of these agents to further exacerbate the patient’s active lung infections. Extracorporeal photopheresis (ECP) would be the best treatment option for this patient due to the severity of his airway obstruction and extrapulmonary involvement, as ECP has demonstrated efficacy and safety in patients with pulmonary, sclerodermatous, and mucosal GvHD.
The investigators suggest the following treatment plan:
- ECP twice weekly for the first month and then twice weekly every other week for 6 to 12 months
- prompt steroid tapering
- pulmonary function tests at least every 4 weeks during the first 3 months
Case presentation: patient 2
The characteristics and initial treatment information for patient 2 are detailed in Figure 2.
Figure 2. Patient 2: characteristics and treatment information*
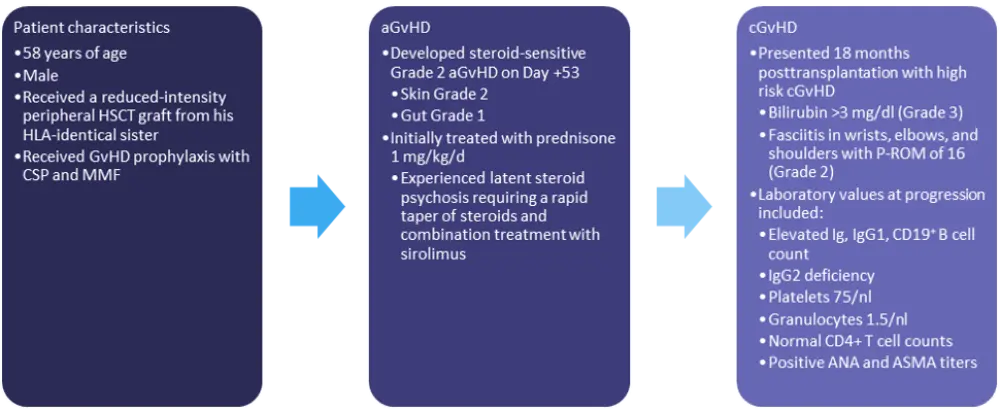
aGvHD, acute GvHD; ANA, antinuclear antibody; ASMA, anti-smooth muscle antibody; cGvHD, chronic GvHD; CMV, cytomegalovirus; CSP, cyclosporine; GvHD, graft-versus-host disease; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplant; Ig, immunoglobulin; MMF, mycophenolate mofetil; P-ROM, photographic range of motion scale.
*Adapted from Wolff, et al.1
Treatment options
Ibrutinib and MMF are likely not the best options for this patient, as he is thrombocytopenic, and ECP takes time to induce a response. For this patient, the combination of ruxolitinib—which may target B cells in addition to T cells and macrophages—with rituximab and low-dose steroids was used. Both ruxolitinib and rituximab have been associated with infectious complications, however, and infection prophylaxis is required.
The investigators suggest the following treatment plan:
- ruxolitinib, rituximab, and low-dose steroids
- Pneumocystis jirovecii and varicella-zoster virus prophylaxis
- continuing antibiotic prophylaxis
- monitoring of blood counts and liver enzymes
Case presentation: patient 3
The characteristics and initial treatment information for patient 3 are detailed in Figure 3.
Figure 3. Patient 3: characteristics and treatment information*
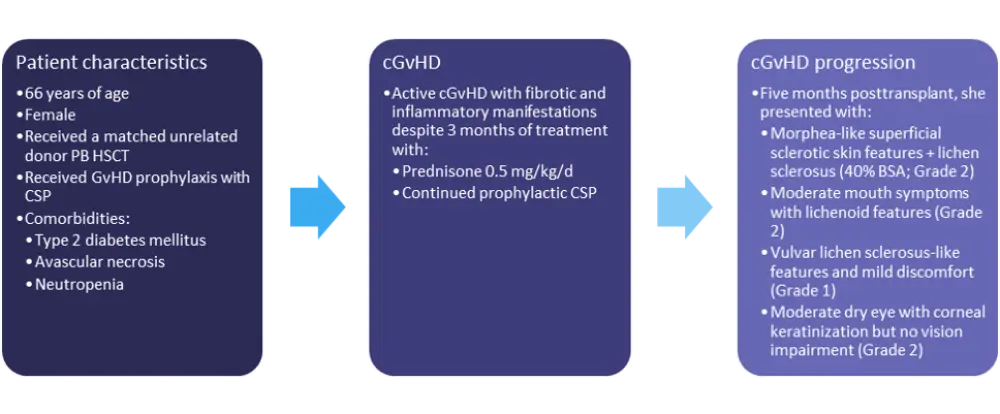
BSA, body surface area; cGvHD, chronic GvHD; CSP, cyclosporine; d, day; GvHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplant; PB, peripheral blood.
*Adapted from Wolff, et al.1
Treatment options
While clinical trial enrollment would be the recommended course of action for this patient, a number of other treatment options are available, including ruxolitinib, ibrutinib, ECP or interleukin-2, rituximab, or MMF with steroids. Any of these treatment options should be given with best supportive care.
Conclusion
As illustrated by these three case studies, there are many options available for the treatment of SR-cGvHD, though only one that is currently approved by the FDA for this indication. The evidence from clinical trials is difficult to interpret, leaving the clinician to select treatment based on experience and on the individual patient’s presentation, including comorbidities. Clinical trial enrollment should be strongly considered in eligible patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

