All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Impact of fungal infections on transplant outcomes: A CIBMTR analysis
Graft-versus-host disease (GvHD) prophylaxis with posttransplantation cyclophosphamide (PTCy) is associated with improved GvHD-free and relapse-free survival. An association between PTCy and viral infections, including cytomegalovirus, non-cytomegalovirus herpes viruses, and community respiratory viruses, has been reported in Center for International Blood and Marrow Transplant Research (CIBMTR) registry studies. However, details relating to certainty of diagnosis, invasive vs superficial infection, treatment information, and antifungal prophylaxis were not captured by these studies.
Recently, Papanicolaou, et al.1 published a CIBMTR cohort study in Transplantation and Cellular Therapy examining the association of haploidentical donor source and/or PTCy with fungal infections. Here, we summarize the key findings.
Study design
This was a CIBMTR registry cohort study comprising 11,964 patients aged ≥2 years undergoing their first hematopoietic stem cell transplantation (HSCT) for acute myeloid leukemia, acute lymphoblastic leukemia, or myelodysplastic syndrome (MDS) between 2012 and 2017. The three cohorts included:
- haplo donor (≥2 antigen/allele mismatch) with PTCy (HaploCy cohort)
- human leukocyte antigen-identical sibling donor with PTCy (SibCy cohort)
- sibling donor with calcineurin inhibitor-based GvHD prophylaxis (SibCNI cohort)
Outcomes of interest included association of donor source and/or PTCy with fungal infection, impact of fungal infection on overall survival, transplant-related mortality (TRM), relapse, and chronic GvHD 2-years post HSCT.
Baseline characteristics
Table 1 shows selected baseline characteristics across the three cohorts.
Table 1. Selected baseline characteristics*
|
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CMV, cytomegalovirus; CsA, cyclosporin; GvHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; MTX, methotrexate; TBI, total body irradiation. |
||||
|
Characteristics, % (unless otherwise stated) |
HaploCy cohort† |
SibCy cohort† |
SibCNI cohort† |
p value‡ |
|---|---|---|---|---|
|
Median age (range), years |
58 (3–78) |
46 (3–75) |
57 (2–78) |
<0.001 |
|
Median donor age (range), years |
36 (9–76) |
45 (4–72) |
54 (2–82) |
<0.001 |
|
Karnofsky/Lansky performance status <80 |
16 |
16 |
12 |
<0.001 |
|
Race/ethnicity |
<0.001 |
|||
|
Caucasian, non-Hispanic |
59 |
59 |
69 |
|
|
Caucasian, Hispanic |
10 |
11 |
8 |
|
|
African-American, non- Hispanic |
17 |
14 |
7 |
|
|
Asian, non-Hispanic |
7 |
7 |
6 |
|
|
Positive CMV status |
72 |
68 |
67 |
0.04 |
|
Disease status |
<0.001 |
|||
|
AML/ALL, early |
41 |
47 |
45 |
|
|
AML/ALL, intermediate |
19 |
19 |
13 |
|
|
AML/ALL, advanced |
13 |
15 |
9 |
|
|
MDS, early |
10 |
6 |
11 |
|
|
MDS, advanced |
17 |
12 |
21 |
|
|
Peripheral blood stem cell grafts |
59 |
67 |
88 |
<0.001 |
|
Myeloablative conditioning |
41 |
55 |
58 |
<0.001 |
|
TBI |
<0.001 |
|||
|
No |
30 |
42 |
73 |
|
|
Yes and >800 cGy |
15 |
20 |
16 |
|
|
Growth factor |
82 |
79 |
24 |
<0.001 |
|
GvHD prophylaxis |
<0.001 |
|||
|
Cyclophosphamide |
100 |
100 |
0 |
|
|
Tac/CsA + MMF ± others |
0 |
0 |
23 |
|
|
Tac/CsA + MTX ± others |
0 |
0 |
77 |
|
|
Median time from diagnosis (range), months |
7 (1–165) |
7 (<1–396) |
5 (1–556) |
<0.001 |
|
Year of HSCT |
<0.001 |
|||
|
2012–2014 |
22 |
22 |
50 |
|
|
2015–2017 |
78 |
78 |
50 |
|
Key findings
Fungal infections
At least one fungal infection 180 days post-HSCT occurred in 7%, 6%, and 4% of patients in the HaploCy, SibCy, and SibCNI cohort, respectively (p < 0.001). Figure 1 shows the proportion of patients for each type of fungal infection across all three cohorts. Fungal infection density scores for yeast and mold are shown in Table 2.
Figure 1. Fungal infection across all cohorts*†
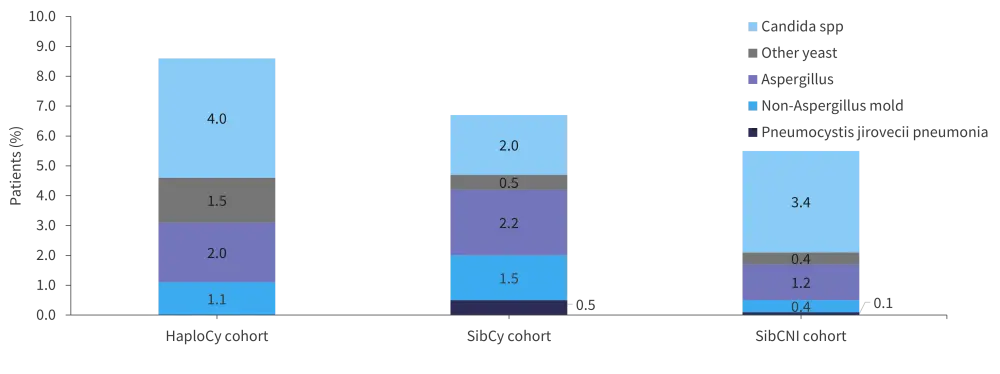
*Data from Papanicolaou, et al.1
†A patient may have had >1 fungal infection.
Table 2. Fungal infection density score*
|
*Data from Papanicolaou, et al.1 |
||||
|
Fungal infection density score |
HaploCy cohort |
SibCy cohort |
SibCNI cohort |
p value† |
|---|---|---|---|---|
|
Yeast |
0.063 |
0.028 |
0.022 |
<0.001 |
|
Mold |
0.036 |
0.047 |
0.019 |
0.009 |
The cumulative incidence of yeast infection was higher in HaploCy cohort compared with SibCy and SibCNI cohort (p < 0.001;Figure 2). The incidence of mold fungal infection was higher in the PTCy cohort vs SibCNI cohort (p = 0.040). This statistical significance was lost when assessing the incidence based on time to neutrophil engraftment and onset of acute GvHD (aGvHD).
Figure 2. Cumulative incidence of fungal infection*
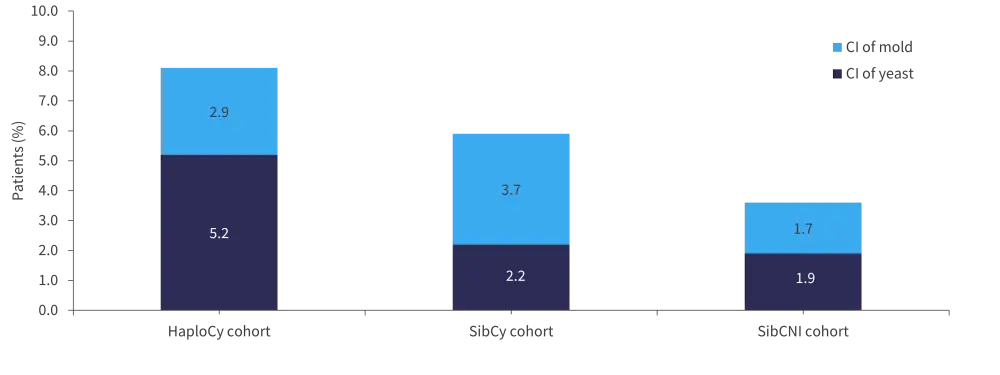
CI, cumulative incidence.
*Data from Papanicolaou, et al.1
Fungal infections and transplant outcomes
Overall survival
Death due to fungal infection occurred in 1.6%, 0.7%, and 0.8% of patients in the HaploCy, SibCy, and SibCNI cohort, respectively. Multivariate analyses showed that presence of fungal infection was associated with increased mortality in the HaploCY and SibCy cohort vs the SibCNI cohort (p < 0.0001). Other factors associated with increased mortality included age >60 years, advanced acute leukemia, high- or very high-risk MDS, aGvHD, and lack of neutrophil engraftment prior to fungal infection (p < 0.0001 each).
TRM
Fungal infections were associated with an increased risk of TRM in both PTCy cohorts compared with SibCNI cohort. Overall, aGvHD, lack of neutrophil engraftment, and high- or very high-risk MDS also contributed to increased risk of TRM (p < 0.0001 each).
Relapse
HSCT performed >6 months after diagnosis and development of aGvHD were associated with a lower risk of relapse (p < 0.0001 each), while use of non-myeloablative or reduced-intensity conditioning regimen (p = 0.0011) and transplantation for high- or very high-risk MDS (p = 0.0014) or advanced acute leukemia (p = 0.0027) were associated with a higher risk of relapse.
Chronic GvHD
A decreased incidence of chronic GvHD was linked to the use of PTCy in the absence of fungal infections; the adjusted hazard ratio for HaploCy and SibCy cohorts were 0.77 (95% confidence interval [CI], 0.57–1.03) and 0.72 (95% CI, 0.56–0.92), respectively.
Figure 3. Association of fungal infections with HSCT outcomes*†
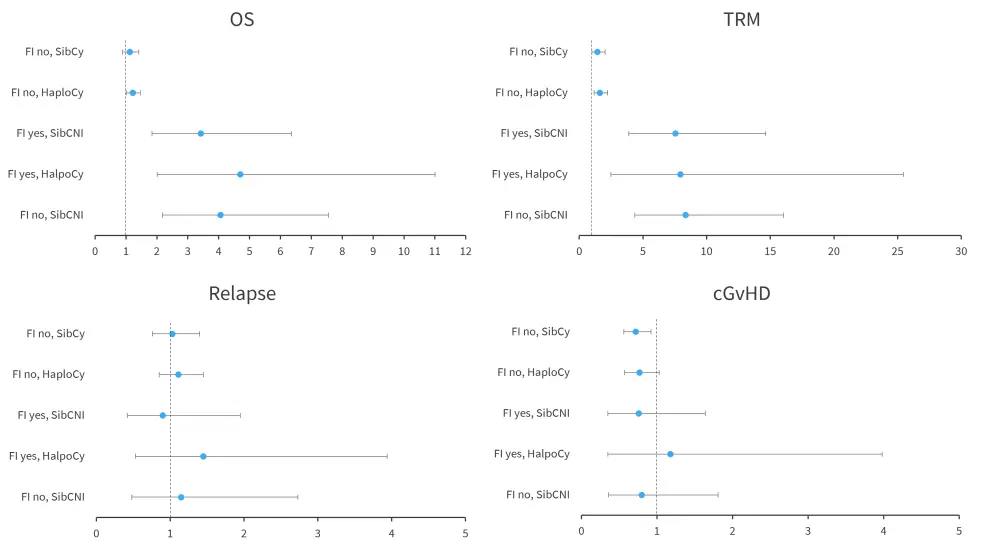
cGvHD, chronic graft-versus-host disease; FI, fungal infection; OS, overall survival; TRM, transplant-related mortality.
*Data from Papanicolaou, et al.1
†Adjusted effect of fungal infection.
Conclusion
This retrospective analysis demonstrated that fungal infection was associated with decreased overall survival and increased TRM at 2 years, irrespective of donor type or use of PTCy. The risk of yeast infection was higher in the HaploCy cohort, and the risk of mold infection was higher in HaploCy and SibCy compared with SibCNI cohort. The authors noted several limitations of this study; including choice of haploidentical graft or PTCy-based prophylaxis, which was made at the discretion of each center; the lack of a standardized definition of fungal infection; and variation in prophylaxis, diagnosis, treatment, and reporting of infections across the three cohorts. Further research focusing on improved strategies for the prevention and treatment of fungal infection posttransplantant is warranted.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

