All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Impact of CD34+ cell dose on patient outcomes after haplo-PBSCT with PTCy
After receiving haploidentical peripheral blood stem cell transplantation (haplo-PBSCT) some patients may also receive posttransplant cyclophosphamide (PTCy) as a graft-versus-host disease (GvHD) prophylaxis. However, it remains uncertain how patients who receive haplo-PBSCT and PTCy are impacted by CD34+ cell dose from their donor graft.
Here, we summarize an article by Maruyama et al.1 published in Blood Cells, Molecules and Diseases evaluating how CD34+ cell dose impacts patient outcomes after haplo-PBSCT with PTCy treatment.
Method1
- A retrospective, single-center study of patients with hematological malignancies who received haplo-PBSCT or matched PBSCT from a human leukocyte antigen-matched related donor at the University of Tsukuba Hospital, Tsukuba, Japan, between May 2011 and May 2022.
- Transplantations were performed using T cell-replete PBSC grafts
- Doses of CD34+ cells and total nucleated cells were defined as the number of cells per recipient body weight, and were measured after graft harvesting
- Patients who underwent haplo-PBSCT received:
- PTCy (50 mg/kg/day) on Days +3 and +5;
- cyclosporine A was administered on Day –1 at a target blood concentration of 500 ng/mL, and tapering began at ~Day +56; and
- mycophenolate mofetil (2,000 mg/day) was administered from Day –1 to Day +30.
- Outcomes measured included:
- median time to neutrophil and platelet count recovery;
- GvHD severity;
- overall survival (OS) at 3 years;
- progression-free survival (PFS) at 3 years;
- GvHD-free relapse-free survival (GRFS) at 3 years;
- cumulative incidence of relapse;
- non-relapse mortality (NRM); and
- complete remission (CR).
- GvHD severity was defined using the Mount Sinai Acute GvHD International Consortium (MAGIC) report and National Institutes of Health classification.
- The receiver operating characteristic (ROC) curve analysis was used to determine the cutoff CD34+ cell dose.
Key findings1
- In total, 111 patients were included in this study.
- 54 patients, with a median age of 47, received matched PBSCT.
- 57 patients, with a median age of 51, received haplo-PBSCT.
- Using the area under the ROC curve, a moderate cutoff value for CD34+ cell dose was established as 3.9 × 106/kg (sensitivity, 66.7%; specificity, 88.1%).
- Patients who received haplo-PBSCT were then divided into two groups to analyze the impact of CD34+ cell dosage which included a haplo-low group (<4.0 × 106/kg CD34+ cells, n = 15) and a haplo-high group (≥4.0 × 106/ kg CD34+ cells, n = 42).
- There were no significant differences observed between the haplo-low and haplo-high groups in patient characteristics, median dose of total nucleated cells, refined disease risk index (p = 0.761), CR status (p = 0.358), or hematopoietic cell transplant comorbidity index score (p = 0.371).
- Patients in the haplo-high group had greater survival outcomes compared with those in the haplo-low group (Figure 1).
Figure 1. Survival outcomes at 3 years after haplo-PBSCT*
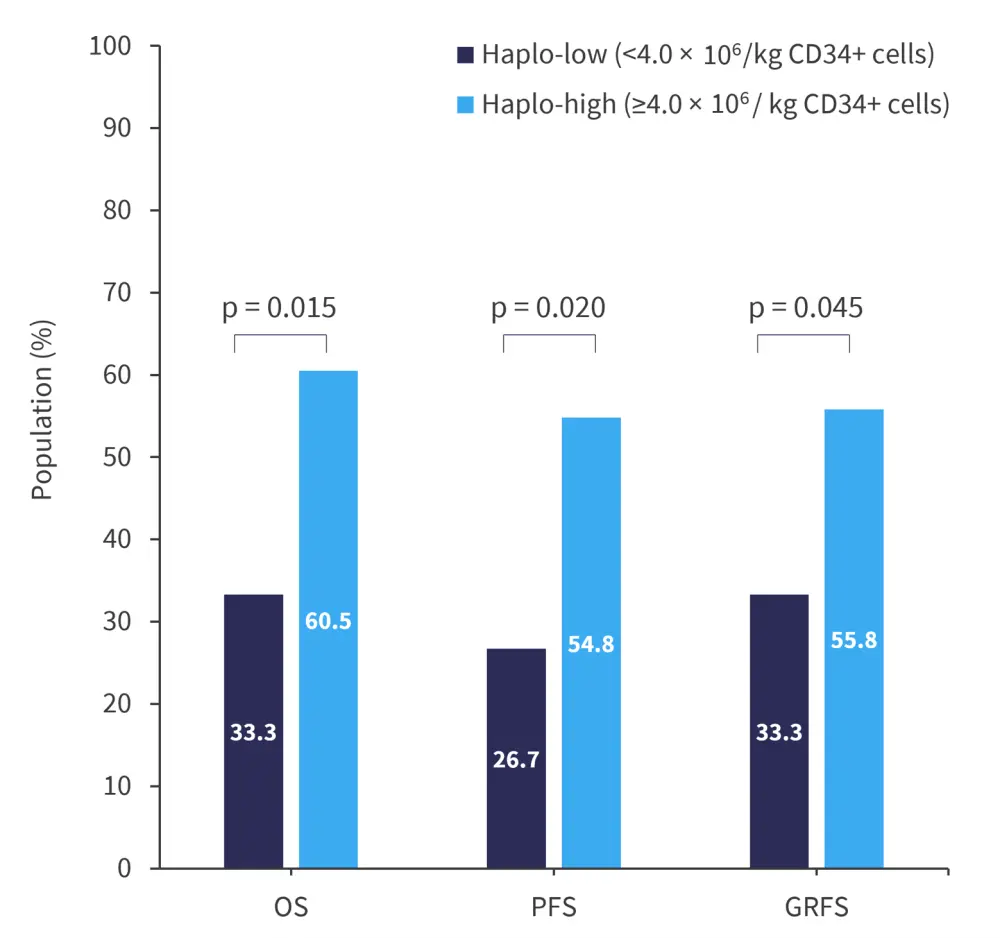
GRFS, graft-versus-host disease-free relapse-free survival; haplo-PBSCT, haploidentical peripheral blood stem cell transplantation; OS, overall survival; PFS, progression-free survival.
*Data from Maruyama, et al.1
- In the haplo-high group, the cumulative incidence of relapse was lower compared with the haplo-low group, 24.1% vs 73.3%, respectively (p = 0.001).
- Table 1 compares additional endpoint data between the haplo-low and haplo-high groups.
- There were no significant differences in the incidence of Grade 2─4 acute GvHD or moderate and severe chronic GvHD.
Table 1. Outcomes of both groups after haplo-PBSCT*
|
|
Haplo-low |
Haplo-high |
p value |
|---|---|---|---|
|
Median time to achieve an absolute neutrophil count of ≥0.5 × 109/L |
17 days |
17 days |
p = 0.832 |
|
Median time to achieve a platelet count of ≥50 × 109/L |
33 days |
29 days |
p = 0.466 |
|
NRM (95% CI), % |
0.0 |
19 |
p = 0.075 |
|
Cumulative incidence of Grade 2-4 aGvHD (95% Cl), % |
40.0 |
16.7 |
p = 0.058 |
|
Cumulative incidence of moderate-to-severe cGvHD (95% CI), % |
13.3 |
17.6 |
p = 0.720 |
|
aGvHD, acute graft-versus-host disease; cGvHD, chronic graft-versus-host disease; CI, confidence interval; haplo-PBSCT, haploidentical peripheral blood stem cell transplantation; NRM, non-relapse mortality. |
|||
- In both univariate and multivariate analysis, disease status and CD34+ cell dose were prognostic for outcomes including OS, PFS, and relapse.
- In multivariate analysis:
- a lower dose of CD34+ cells (<4.0 x 106/kg) correlated with disease relapse (hazard ratio [HR], 4.51; 95% confidence interval [CI], 1.66─12.2; p = 0.003); and
- a non-CR correlated with shorter OS (HR, 2.63; 95% CI 1.13─6.14; p = 0.025), shorter PFS (HR, 2.63; 95% CI 1.23─5.63; p = 0.013), and a higher relapse rate (HR, 2.97; 95% CI, 1.37─6.43; p = 0.006).
- In multivariate analysis:
|
Key learnings |
|---|
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


