All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Establishing the optimal co-receptor signaling domain for chimeric antigen receptor Treg cell therapy
Regulatory T cells (Tregs) play an important role in mediating immune tolerance after stem cell transplantation, and a reduction in Tregs has been associated with the development of graft-versus-host disease (GvHD). Treg reconstitution is being actively investigated for the prevention of GvHD, as recently presented on the GvHD Hub, and although most clinical trials have used polyclonal Tregs, antigen-specific Tregs engineered using chimeric antigen receptors (CARs) may be a more potent approach.1
CAR T cell therapy, which is showing great promise as a cancer immunotherapeutic, was originally developed using conventional T cells. Tregs have different signaling requirements to conventional T cells. Although multiple studies have used CAR Tregs with the CD28 or 4-1BB domains, the most optimal co-receptor signaling domain for potent CAR Treg function is yet unclear.2 In this study, published in Science Translational Medicine by Nicholas Dawson and colleagues, the functional effects of different co-receptor signaling domains on antigen-specific CAR Tregs were investigated both in vitro and in an in vivo GvHD model.2
Methods
CARs were designed according to the schematic shown in Figure 1.
Figure 1. CAR construct design2
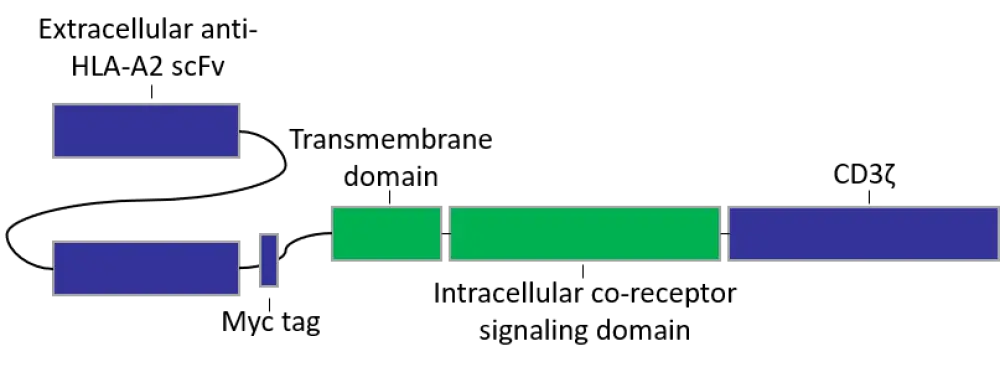
HLA, human leukocyte antigen; scFv, single chain variable fragment.
CAR constructs were cloned into a lentiviral vector that encoded a truncated nerve growth factor receptor (NGFR) as a transduction marker. Flow cytometry-isolated CD4+ CD25+ CD127− or CD4+ CD25+ CD127− CD45RA+ human Tregs were transduced. Seven days later, NGFR+ cells were isolated by magnetic sorting (CAR Tregs).
Function of the various constructs was assessed in vivo using a well-established HLA-A2 mismatched xenogeneic GvHD mouse model. Immunodeficient NOD/SCID gamma (NSG) mice were injected with 107 HLA-A2+ peripheral blood mononuclear cells (PBMCs) and either 2.5 × 106 CAR Tregs (low ratio) or 5 × 106 CAR Tregs (high ratio). Control groups were injected with either saline or PBMCs without CAR Tregs. The ability of the CAR Treg variants to provide protection from GvHD was assessed by survival and GvHD score (a scale from 0–3 based on weight loss, skin integrity, fur maintenance, activity, and deteriorating posture).
In vitro activity of CAR constructs was evaluated in several ways:
- Expression of Treg activation markers and cytokine production;
- Suppression of CD4+ and CD8+ T cells;
- Assessment of CD86 and CD80 expression on dendritic cells (DCs); and
- Treg transcriptome analysis.
Results
Generation of CAR variants
In all, nine different CAR Treg variants were tested against an existing CAR construct comprising an intracellular CD28 domain and extracellular single-chain variable fragment (scFv) specific for the human leukocyte antigen (HLA)-A2 (CD28wt CAR Treg). The nine examined CARs consisted of alternative co-stimulatory domains selected from CD28 or tumor necrosis factor receptor (TNFR) family proteins based on their functional relevance and success in conventional T cells (Table 1).
Table 1. Signaling domains for CAR Treg variants2
|
Construct |
Transmembrane domain |
Intracellular co-receptor signaling domain |
Rationale for selection |
|
CAR T, chimeric antigen receptor T cells; CTLA-4, cytotoxic T lymphocyte-associated protein 4; GITR, glucocorticoid-induced TNFR-related protein; ICOS, inducible T cell costimulatory; IL-10, interleukin-10; PD-1, programmed cell death protein 1; PIK3, phosphoinositide 3-kinase; TNFR, tumor necrosis factor receptor. |
|||
|
CD28wt |
CD28 |
CD28 |
Acute anti-tumor activity |
|
CD28mut |
CD28 |
CD28 (Y173F) |
Mutant with reduced PI3K pathway activity, likely beneficial for Treg function |
|
ICOS |
ICOS |
ICOS (CD278) |
Important for Treg survival, may be involved in IL-10 production |
|
CTLA-4wt |
CD28 |
CTLA-4 (CD152) |
Essential for Treg function |
|
CTLA-4mut |
CD28 |
CTLA-4 (Y165G) |
Mutant with increased cell surface expression |
|
PD-1 |
CD28 |
PD1 (CD279) |
Required for peripheral Treg production and maintenance |
|
OX40 |
OX40 |
OX40 (CD134) |
Promote Tregs in some contexts |
|
GITR |
GITR |
GITR (CD357) |
Promote Tregs in some contexts |
|
4-1BB |
4-1BB |
4-1BB (CD137) |
Important for Treg and CAR T cell survival |
|
TNFR2 |
TNFR2 |
TNFR2 (CD120b) |
Stimulates Treg proliferation and expression |
- Cell surface expression following transfections in human embryonic kidney 293T cells was similar for most CAR constructs. However, expression was higher for the cytotoxic T lymphocyte-associated protein 4 mutant (CTLA-4mut) when using the CD28 transmembrane domain, and the glucocorticoid-induced TNFR-related protein (GITR) when using the native domain.
- When examining isolated CAR Treg cells, there was little difference between the CAR variants in their surface expression frequency, with the CTLA-4wt variant showing a lower CAR expression compared with the CD28wt CAR. In terms of mean fluorescence intensity, CD28wt and CD28mut CAR constructs showed a consistently higher expression.
Suppression of Tregs in an in vivo GvHD model
- Mice receiving CAR Tregs at low ratios had a significantly superior survival when injected with the CD28wt CAR Tregs than any other CAR Treg variant (CD28mut, p = 0.014; ICOS, p = 0.016; CTLA-4wt, p = 0.014; PD1, p = 0.016; OX40, p = 0.011; GITR, p = 0.014; 4-1BB, p = 0.011; TNFR2, p = 0.016).
- For mice receiving CAR Tregs at high ratios, the CD28mut and ICOS CAR Tregs provided similar protection from GvHD as the CD28wt CAR Tregs.
- Regardless of cell-injection ratio, CARs encoding for TNFR family co-receptors provided poor protection and poor GvHD scores.
- These observations were consistent with the T cell engraftment and proliferation of the HLA-A2+ cells; mice injected with CD28wt CAR Tregs had the slowest increase in CD45+ cell engraftment and the lowest levels of HLA-A2+ cells after 7 days.
- At Day 7, the number of circulating CD28wt CAR Tregs was higher, in both proportion and absolute number, than any other CAR Treg variant and was the only detectable circulating CAR Treg by Day 14.
Activation of Tregs and production of cytokines
CAR Tregs were stimulated with HLA-A2-expressing K562 cells to assess their ability to activate Tregs.
- Expression of T cell activation markers CD69 and CD71 was highest for CD28wt (75% CD69+CD71+ cells) and CD28mut and lowest for PD-1 CAR Tregs (3% CD69+CD71+ cells).
- Similarly, the expression of Treg-specific effector molecules (latency-associated peptide [LAP], glycoprotein-A repetitions predominant [GARP], CD39, CTLA-4) was highest for CD28wt (95% LAP+GARP+ and 82% CTLA-4+CD39+ cells) and CD28mut and lowest for PD-1 CAR Tregs (6% LAP+GARP+ and 8% CTLA-4+CD39+ cells).
- All stimulated CAR Tregs had a low level of cytokine production in comparison to conventional T cells expressing CD28wt CAR. None produced detectable levels of interleukin (IL)-2, IL-4, or TNF-α; however, the CD28wt and CD28mut CAR Tregs produced interferon (IFN)-γ IL-17A, IL-10, and IL-6.
High levels of the transcription factor forkhead box protein 3 (FOXP3) and demethylation of the Treg-specific demethylation region (TDSR) are distinct characteristics of Tregs, while Helios expression is considered important for Treg stability. The long-term effect of Treg activation was assessed by monitoring cell number, expression of FOXP3 and Helios, and TDSR methylation over 12 days.
- Only CD28wt, 4-1BB, and TNFR2 CAR Tregs proliferated after HLA-A2 stimulation in vitro.
- There was no differential expression of FOXP3, however 4-1BB and TNFR2 CAR Tregs caused reduced Helios expression.
- Following 12 days of HLA-A2 Treg stimulation, TNFR2 CAR Tregs also showed a significant increase in TDSR methylation compared with the other constructs.
- While most CAR Tregs, including CD28wt, promoted effector memory Treg expansion, 4-1BB and TNFR2 CAR Tregs induced central memory T cell expansion and PD-1 CAR Tregs increased the proportion of naive T cells.
- Taken together, these results indicate that proliferation and effector memory expansion is required for CAR Tregs to function effectively, but excessive or quick proliferation is harmful.
Suppression of T cells and dendritic cells in vitro
Evidence suggests that antigen-presenting cells play a key role in T cell suppression, so in addition to measuring suppression of CD4+ and CD8+ cells, the authors evaluated expression of co-stimulatory molecules on DCs. CAR Treg stimulation was performed via a combination of anti-CD3/-CD28 bead exposure and HLA-A2 CAR Treg in vitro pre-stimulation. These in vitro results were compared with data from the in vivo GvHD model.
- CD28wt CAR Tregs suppressed CD4+ and CD8+ T cells more potently than other variants (p values ranged from < 0.05 to < 0.001).
- The expression of CD80 and CD86 on DCs was more greatly reduced by CD28wt CAR Tregs than the other variants and the 4-1BB- and TNFR2-encoding CARs had the lowest capability for CD80 suppression.
- A significant correlation was found between Day 28 in vivo GvHD score and in vitro CD80 suppression on DCs (p = 0.0473), supporting a role for DCs in Treg suppression.
- It is worth noting, however, that the in vitro assays were not entirely predictive of the in vivo results. No correlation was found between CAR Treg function in the in vivo model, according to Day 28 GvHD score, and suppression of CD4+ or CD8+ T cells or CD86 DC expression.
Modulation of Treg gene expression
- CD28wt CAR Tregs promoted enrichment of genes targeting transcription factors associated with DNA replication and cell proliferation, including minichromosome maintenance protein (MCM) complex, MYB, and E2F4, as well as RELA transcription factors.
- CD28wt CAR Tregs had higher expression of genes associated with cell cycle and division, and the importance of cell cycling in CD28wt CAR Treg function was indicated by increased expression of genes associated with mTORC1 signaling, G2-M checkpoint, and Myc targets.
- Conversely, the 4-1BB and TNFR2 CAR Tregs, which proliferated in response to A2 but did not maintain Helios expression, had a higher expression of genes associated with inflammatory cytokine response pathways, including IFN and TNF, when compared to CD28wt CAR Tregs.
- A similar gene expression pattern was found for Tregs stimulated with CD28wt CAR and those activated via anti-CD3/CD28 stimulation, indicating that, at least for this type of CAR construct, stimulation replicates endogenous anti-CD3/CD28 cross-linking TCR/CD28 signaling activation.
Study limitations
Although CAR Tregs showed activity in the in vivo GvHD mouse model, it is unknown how well these results translate to human trials. The authors acknowledge that the xenogeneic system does not represent a normal immune response and recommend that the performance of CAR Tregs should be further tested in syngeneic models.
Conclusion
This comprehensive study supports the use of the CD28wt co-stimulatory domain for potent CAR Treg function. There was a clear survival advantage for CD28wt CAR Tregs in vivo, which could not be entirely predicted by the in vitro results. Survival benefit and the ability of CD28wt CAR Tregs to proliferate in response to HLA-A2 was supported by transcriptome analysis showing enrichment of genes involved in DNA replication and cell proliferation pathways. The findings of this study might be valuable for CAR design optimization for future Treg therapies.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

