All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Editorial theme | Clinical manifestations and pathophysiology of chronic GvHD
In the coming weeks, the GvHD Hub will explore the clinical presentation and development of chronic graft-versus-host disease (cGvHD), as well as the current and future directions of its treatment. We will be considering the latest updates from recent meetings, including the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition. In this first article, we review clinical manifestations, classification, and immune mechanisms underlying the development of cGvHD.
Incidence and clinical manifestations
cGvHD represents the major cause of nonrelapse mortality and morbidity post allogeneic hematopoietic stem cell transplantation (allo-HSCT).1
The incidence of cGvHD in allogeneic recipients is 10─50%, with a higher incidence if peripheral blood is used as the graft source. Once cGvHD occurs, most patients require prolonged treatment with multiple lines of therapy.2
Early manifestations of cGvHD are characterized by inflammatory skin rash, oral sensitivities or dryness, or dry, irritated eyes; these symptoms are relatively easy to control with standard corticosteroid-based immunosuppression. Other, less common but more difficult to control, manifestations include skin sclerosis or fasciitis, bronchiolitis obliterans syndrome, oral ulcers unresponsive to local therapies, severe dry eyes, serositis, and gastrointestinal involvement.3
Prior to 2005, cGvHD was defined as any clinical alloimmunity occurring later than 100 days post allo-HSCT. However, diagnostic features can be seen before, and may occur with those seen in acute GvHD (aGvHD). At the 2005 National Institutes of Health (NIH) Consensus Conference, this definition was reviewed, the time restriction abolished, and a new category added called ‘overlap cGVHD’—when concurrent aGvHD and cGvHD are present.3
Diagnostic features sufficient to establish the diagnosis of cGvHD include lichen planus-like lesions or lichen sclerosis, poikiloderma, sclerosis, esophageal webs, or bronchiolitis obliterans.3
Based on number and severity of involved organs, cGvHD can be classified as mild, moderate, or severe (Table 1).3
Table 1. National Institutes of Health (NIH) severity scoring3
|
ADL, activities of daily living; BSA, body surface area; FEV1, forced expiratory volume in one second. |
|
|
Mild |
1 or 2 organs or sites (except lung) with Score 1 Mild oral symptoms, no decrease in oral intake Mild dry eyes, lubricant eyedrops ≤3 times per day |
|---|---|
|
Moderate |
≥3 organs with Score 1 ≥1 organ/site with Score 2 19─50% body surface area involved or superficial sclerosis Moderate dry eyes, eyedrops > 3 times per day or punctal plugs Lung score 1 (FEV1 60─79% or dyspnea with stairs) |
|
Severe |
≥1 organ/site with Score 3 >50% BSA involved Deep sclerosis, impaired mobility, or ulceration Severe oral symptoms with major limitation in oral intake Severe dry eyes affecting ADL Lung score 2 (FEV1 40─59% or dyspnea walking on flat ground) |
Phases of cGvHD pathophysiology
The proposed mechanism for the initiation and development of cGvHD involves early inflammation caused by tissue injury, dysregulated immunity, and aberrant tissue repair and fibrosis (Figure 1).4
Figure 1. Pathophysiology of cGvHD4
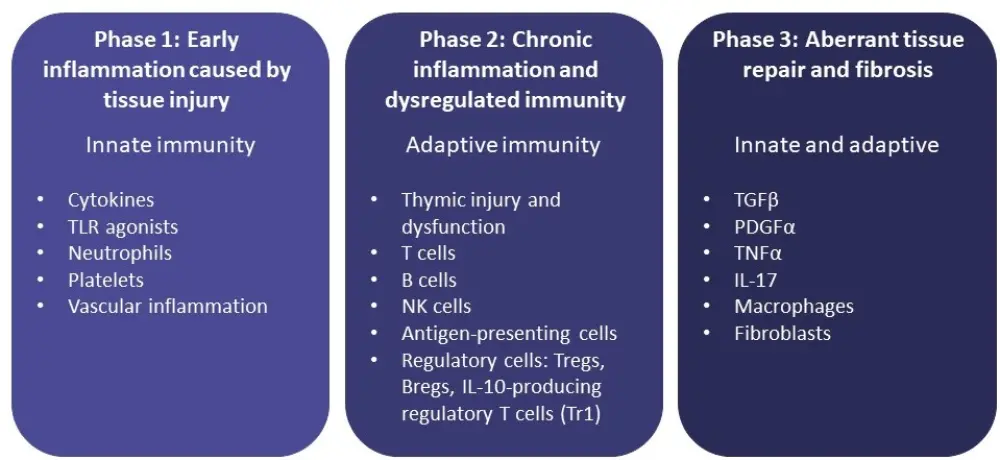
Phase 1
Tissue damage results in the following5:
- Translocation of bacteria and fungi and their products through epithelial surfaces, leading to the release of pathogen-associated molecular patterns;
- Release of damage molecules, which trigger inflammasome pathways and propagate inflammation; and
- Induced activation of T cells, caused by the triggering of the innate immune system.
Phase 2
- Alloreactive B cells and T cells are primed by antigen-presenting cells, inducing expansion and polarization toward type 1, type 2, and type 17 helper T cells (Th1, Th2, and Th17).5
- Inflammation is maintained by
- autoreactive and alloreactive CD4+ T cells with the production of interleukin-17A, and
- activated follicular helper T cells producing interleukin-21.5
- Thymic injury, induced by alloreactive T cells, causes impaired positive and negative selection and loss of regulatory-cell populations, including regulatory T cells, regulatory B cells, regulatory natural killer cells, and invariant natural killer T cells.5
Phase 3
- Activated macrophages produce platelet-derived growth factor α (PDGF-α) and transforming growth factor β (TGF-β). This leads to the activation of fibroblasts, which results in production of extracellular matrix proteins and their deposition, eventually leading to sclerosis.5
- Plasma cells, fueled by B-cell activating factor, produce isotype-switched immunoglobulin, resulting in pathogenic immunoglobulin deposition in various organs, which in turn contributes to organ damage and fibrosis.5
cGvHD and graft-versus-tumor effect
Alloimmune responses are responsible for the development of GvHD, but they also mediate graft-versus-tumor (GvT) effect, which represents one of the advantages of allo-HSCT over autologous HSCT for the treatment of hematologic malignancies.6 While cGvHD is associated with improved overall survival because of the lower relapse rate caused by the presence of a potent GvT effect, more severe cGvHD is associated with an increased risk of mortality.7
Thus, considering the importance of the GvT effect and the higher risk of mortality associated with severe cGvHD, novel therapeutic approaches are needed to preserve the benefits of the GvT effect while controlling cGvHD severity in recipients of allo-HSCT.7
Impact of the stem cell source on cGvHD
The source of stem cells also plays a role in the development of cGvHD.7 Patients transplanted using peripheral blood as the stem cell source have a higher incidence of cGvHD compared with patients transplanted with bone marrow or cord blood stem cells.7 The use of peripheral blood as a stem cell source was associated with increased incidence of cGvHD after both haploidentical HSCT and matched unrelated donor HSCT.
Conclusion
A better understanding of the events leading to cGvHD, and novel approaches able to preserve the benefits of the GvT effect while controlling cGvHD severity, are needed for the development of new targeted therapies, which may improve the clinical outcomes for patients with cGvHD.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

