All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Developments in ocular GvHD
Do you know... Which ocular examination finding has been shown to be associated with poor vision outcomes in ocular GvHD?
Chronic graft-versus-host disease (GvHD) is a significant complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT), with major organs involved, such as the skin, liver, gastrointestinal tract, lungs, and eyes.1
Ocular involvement, seen in up to 60–90% of patients with chronic GvHD, usually presents with symptoms of severe dry eye, discomfort, and an associated reduction in quality of life.1,2 Whilst the pathophysiology is not yet fully understood, an inflammatory environment thought to be a result of complex interactions between donor immune cells and host histocompatibility antigens is responsible for impaired ocular epithelial integrity and destruction and fibrosis of the conjunctiva and lacrimal gland.2 Severe vision loss secondary to corneal involvement can occur if it is not treated in time.1
In this article, we consider recent interesting developments in ocular GvHD (oGvHD), including recent research by Shen, et al.1 to identify potential tear cytokine biomarkers that may aid diagnosis and indicate the severity of oGvHD, as well as the investigation by Yoon, et al.2 of long-term clinical outcomes and predictive factors associated with poor vision in patients with oGvHD. We also consider the study by Gehlsen, et al. 3 on desiccating stress on transplant wards and whether modifying air humidity offers the opportunity to reduce rates of oGvHD.
Tear biomarkers: The potential to improve diagnosis?1
Early diagnosis of chronic oGvHD is challenging given patients frequently experience other more life-limiting complications that prevent easy access to specialist ophthalmology clinics. In addition, the symptoms and clinical signs of oGvHD are not overly disease specific. Tear protein analysis could offer non-invasive and easily repeatable testing to aid diagnosis and assess disease severity; therefore, this is a current research hot topic.
T cells are a well-documented driver of alloreactivity in oGvHD. The aim of the study by Shen, et al.1 was to consider the less well-understood role of B cells and to identify sensitive biomarkers of oGvHD. Tear analysis was undertaken for patients diagnosed with chronic oGvHD (18 patients, 27 eyes) in accordance with the National Institute of Health (NIH) consensus compared with patients with dry eye disease (11 patients, 22 eyes) as a control group (Figure 1).
Figure 1. Flow diagram showing patient selection and exclusion in the study by Shen, et al.*
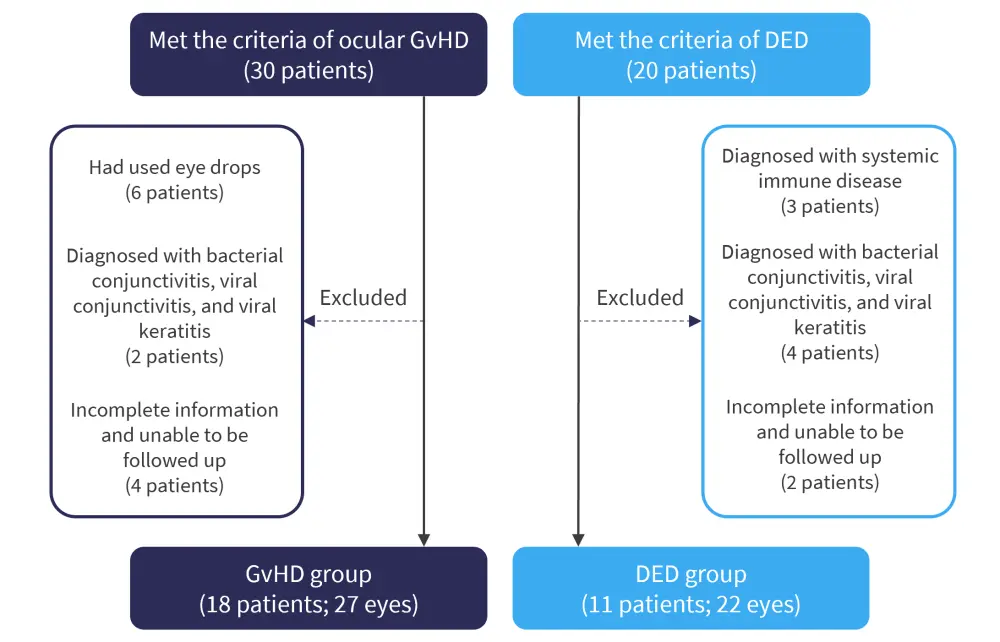
DED, dry eye disease; GvHD, graft-versus-host disease.
*Adapted from Shen, et al.1
In addition to the tear fluid analysis of 29 cytokines, symptoms were assessed with the Ocular Surface Disease Index (OSDI) questionnaire and an objective ocular examination was performed (intraocular pressure, visual acuity, fluorescein tear film break-up time, and corneal fluorescein standing).
Statistically significant differences were seen in 22 of the cytokines measured between the oGvHD and dry eye disease groups. Following further correlation analysis with ocular surface parameters, eight cytokines showed correlation and thus biomarker potential: interleukin (IL)‑2, IL‑6, IL‑8, intercellular adhesion molecule-1 (ICAM‑1), CD62E, E‑selectin, neuropilin‑1, and B‑cell activation factor (BAFF). Furthermore, in patients with oGvHD, B cell-associated cytokines were characterized by increased BAFF and decreased proliferation-inducing ligand (APRIL), and APRIL/BAFF showed superior diagnostic capabilities. This suggests that B cells may also play an important role in oGvHD and provides direction for future study.
There were some limitations of the study, most notably Shen et al. comment on selection bias, given patients with oGvHD invariably present to the specialist ophthalmology clinic (from which they were enrolled) with advanced symptoms, and the small study size. This study paves the way for large-scale prospective studies to further illuminate the role of tear cytokine biomarkers and the mechanism of B-cell involvement in oGvHD.
Predicting long-term clinical outcomes and poor final visual prognosis in oGvHD2
Criteria proposed by the NIH consensus and International Chronic oGVHD Consensus Group (ICOGCG), whilst helpful diagnostically, provide no meaningful prognosis of vision outcomes. Given the challenge of treating sight-threatening complications of oGvHD, Yoon and colleagues2 conducted a study considering risk factors for long-term clinical outcomes and poor final visual prognosis in patients with oGvHD, with the hope that identifying risk factors associated with severity and poor outcomes will aid early clinical management.
This retrospective cohort study included 94 patients with chronic oGvHD (classified as either severe [n = 25] or non-severe [n = 69]) who had been referred for ophthalmological assessment and followed up for at least a year. Scores were assigned for oGvHD severity according to the ICOGCG and NIH scoring systems.
Analysis revealed myelodysplastic syndromes (MDS) were a significant predictive factor of severe oGvHD. The authors consider this association could be due to the lack of other treatment options for this disease group, thus hematologists look to enhance graft-vs-leukemic effect over the potential complications. A subset analysis of these patients revealed they tended to have a greater number of organs involved in their GvHD.
Whilst there was no direct correlation between systemic GvHD grade and oGvHD, lung GvHD emerged as a risk factor for severe oGvHD. It is possible that this is because the pathophysiological mechanism of injury is comparable, with the conjunctiva possibly mirroring lung mucosal membranes.
No history of systemic calcineurin inhibitor use was also identified as a significant predictive factor of severe oGvHD. The initiation of topical calcineurin inhibitor cyclosporine pre-transplant has been shown in several previous studies to reduce the inflammatory response in the lacrimal glands. Interestingly, the findings in this study would suggest that systemic immunosuppressants in the early phase of allo-HSCT may be delivered to ocular tissues and help prevent ocular complications.
The presence of conjunctival scarring and persistent epithelial defects seen on ophthalmological examination were significantly associated with poor vision outcomes. Further, significant correlation was seen between the final best-corrected visual acuity and both the NIH and ICOGCG scoring systems. At 5 years, rates of poor vision outcomes were significantly higher in the severe oGvHD group and 5-year survival rates were significantly lower.
Interestingly, this is the first study to propose long-term disease activity as either persistent or episodic. In comparison with the non-severe group, the disease course in the severe group tended to be persistent, and in episodic disease, flare-up episodes were common and more frequent.
The study did have a number of limitations in that it is a small study that was retrospective in nature. As a result of limited follow-up, patients with systemic GvHD but no ocular GvHD were not included. Ocular status pre-HSCT and medication received were also not controlled in this study.
Desiccating stress on the hospital transplant ward: A modifiable risk for oGvHD3
A constant dry environment is well known to cause desiccation (drying) of the ocular surface. This desiccating stress induces dry eye symptoms and dry-eye disease.3 Given patients undergoing allo‑HSCT are in a highly climate-regulated environment and are thus potentially exposed to desiccating stress, Gehlsen and colleagues3 studied desiccating stress as a potentially modifiable risk factor for chronic oGvHD.
In their retrospective cohort study of 444 consecutive patients undergoing allo-HSCT, data were collected on relative humidity (rH%) on the transplant ward, with an average humidity taken from the time of allo-HSCT until 30 days after transplantation, with four humidity risk classes proposed: class I, ≤30 %rH; class II, 31–40 %rH; class III, 41–50 %rH; and class IV, >50 %rH. Patients underwent detailed ophthalmological examination for oGvHD at 3, 6, 12 and 18 months. Given the retrospective nature of the study, not all data were available for the 444 patients followed up for a maximum of 5.8 years after transplant (Figure 2).
Figure 2. Patients included in the study by Gehlsen et al. on the effect of desiccating stress on ocular GvHD*
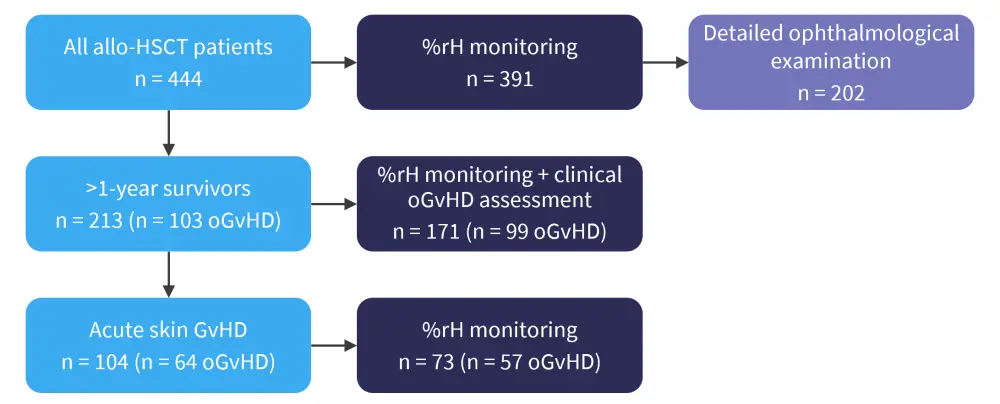
%rH, percentage relative humidity; allo-HSCT, allogeneic hematopoietic stem cell transplantation; oGvHD, ocular graft-versus-host disease.
*Adapted from Gehlsen, et al.3
As shown in Figure 3, significant differences in the incidence of oGvHD were seen between the humidity risk classes, with significant correlation between oGvHD and lower %rH. Patients who underwent allo-HSCT and were in risk class I had a 20% increased risk of oGvHD (relative risk [RR], 1.2) in comparison with those in risk classes II and III. This risk increased to 140% (RR, 2.4) compared to those in humidity class IV. With each increase of the average humidity by one percent, the RR of oGvHD was decreased by 4%, or by 37% with each risk class. Interestingly, no correlation was seen with the severity of oGvHD or the time of diagnosis. In keeping with previous research, correlation between acute skin GvHD and ocular GvHD was observed.
Figure 3. Incidence of ocular GvHD by relative humidity class for 151 1-year survivors*
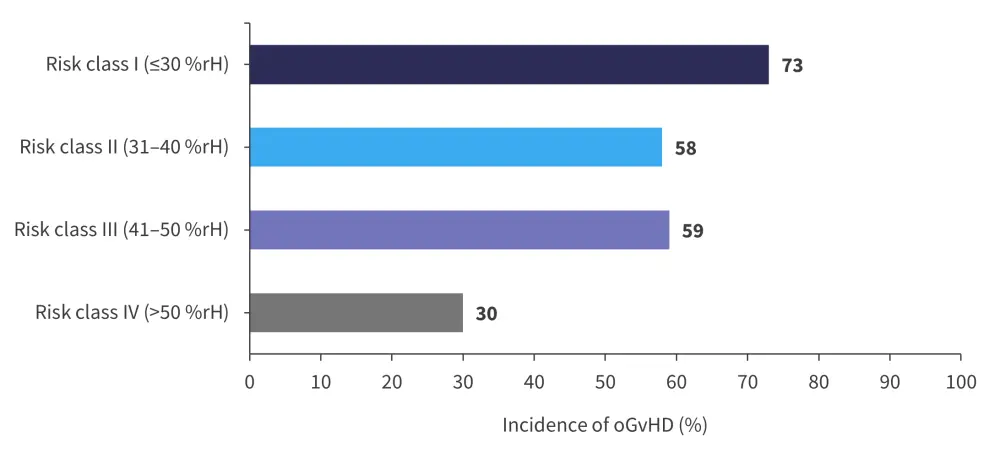
%rH, percentage relative humidity; oGvHD, ocular chronic graft-versus-host disease.
*Adapted from Gehlsen, et al.3
Despite the limitations of this study, such as its retrospective design and limited analysis of patient subgroups given low patient numbers, and the potential for confounding effects between adverse environmental conditions and skin GvHD, low humidity (particularly ≤30 %rH) emerged as an independent risk factor for oGvHD. These clinically relevant findings suggest adjusting air humidity on transplant wards has the potential to reduce the rate of oGvHD.
Conclusion
These recent studies offer exciting advances in oGvHD. Analysis of tear biomarkers may provide a non-invasive method of monitoring oGvHD. Whereas the work by Yoon, et al. highlights that patients with MDS, lung GvHD, or with no history of calcineurin inhibitor use may be at risk of severe oGvHD and should be monitored accordingly. Finally, the importance of monitoring and adjusting relative air humidity on transplant wards to reduce the incidence of oGvHD was reported.
With future research to build on these findings, the combination of improved diagnostic ability and better prediction of long-term outcomes will hopefully see improved management of this allo-HSCT complication that has a significant effect on quality of life. Importantly, modification of the closely monitored environment on the transplant ward may offer potential to reduce the rates of oGvHD.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

