All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
De-escalation of posttransplant cyclophosphamide in preventing acute graft-versus-host disease
Hematopoietic cell transplantation (HCT) provides a valid option to cure or manage blood cancers. One of the major complications following transplant is acute graft-versus-host disease (aGvHD) which is accompanied with high mortality rates. To prevent aGvHD, posttransplant cyclophosphamide (PTCy) is commonly used at a standard daily dose of 50 mg/kg on Days 3–4 following transplant.
In animal models, a daily dose of 25 mg/kg on Days 3–4 was shown to be superior to a 50 mg/kg/day dosing regimen in preventing GvHD1, and a single daily dose of 25 mg/kg given at Day 4 was equivalent to the same dose given at Days 3–4.2
Meredith McAdams et al. investigated PTCy dose de-escalation in preventing GvHD in a phase I/II study (NCT03983850) and presented their results during the 2021 Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR.3
Study design
The aim of this study was to evaluate if de-escalated PTCy dosing following human leukocyte antigen (HLA)-haploidentical HCT would preserve protection against severe aGvHD while allowing for reduced toxicity, faster engraftment, and more robust immune reconstitution leading to enhanced immunity against infections. The treatment plan is depicted in Figure 1.
- Three dose levels (DL) in a 3+3 dose de-escalation design were tested
-
- DL1: 50 mg/kg/day (Days 3–4)
- DL2: 25 mg/kg/day (Days 3–4)
- DL3: 25 mg/kg/day (Day 4)
- Primary endpoint and dose-limiting toxicity were Grade 3–4 aGvHD events
Figure 1. Treatment schema3
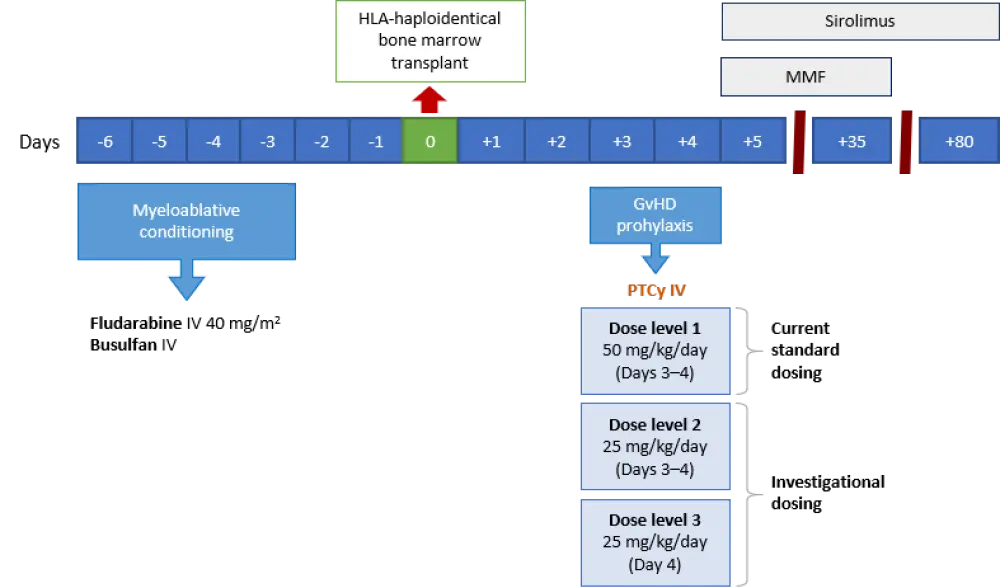
GvHD, graft-versus-host disease; HLA, human leukocyte antigen; IV, intravenous; MMF, mycophenolate mofetil; PTCy, posttransplantat cyclophosphamide.
Patients
The number of patients was 19. Baseline characteristics among different dose levels are presented in Table 1.
- The study population was young with a median age of 27 years
- The median Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) score was 4
- Heavily pretreated population with a median number of prior lines of therapy of 3
Table 1. Baseline characteristics by dose level3
|
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; B-ALL, B-cell ALL; BMT, bone marrow transplant; CR, complete remission; DL, dose level; DLBCL, diffuse large B-cell lymphoma; DRI, disease risk index; HCT-CI, hematopoietic cell transplantation comorbidity index; HL, Hodgkin Lymphoma; MDS, myelodysplastic syndromes; T-ALL, T-cell ALL. |
|||
|
Characteristic, n |
DL1 (n = 5) |
DL2 (n = 7) |
DL3 |
|---|---|---|---|
|
HCT-CI score Low risk (0) Intermediate risk (1−2) High risk (≥3) |
― ― 5 |
― 3 4 |
2 1 4 |
|
Diagnosis B-ALL AML HL DLBCL MDS |
3 1 ― 1 ― |
4* 2 1 ― ― |
2 3† 1 ― 1 |
|
Disease status at BMT CR1 CR2/3 Refractory |
3 1 1 |
4 2 1 |
4 2 1 |
|
Revised DRI Low Intermediate High/very high |
― 3 2 |
― 4 3 |
1 2 4 |
Results
Median follow-up for survivors was 294 days (range, 40–473).
- aGvHD
- All aGvHD events seen with DL2 or DL3 were Grade 1 or 2, and limited to the skin
- There was one Grade 4 aGvHD event seen with DL1
- Engraftment
- Lower PTCy (DL2) dosing was associated with significantly faster neutrophil and platelet engraftment compared with standard dosing (p ≤ 0.01 and p ≤ 0.05, respectively)
- There was a trend for faster engraftment with DL3
- One patient had poor graft function in the DL1 arm and one patient at each of DL2 and DL3 arms had split chimerism
- One patient in DL2 had primary graft failure and one patient in DL3 experienced relapse before engraftment
- Mucositis
- All patients in the DL1 arm developed Grade 3 mucositis, while in the DL2/3 arms some patients had only Grade 2 mucositis
- Duration of Grade 2/3 mucositis was significantly lower with DL2
- Duration of Grade 3 mucositis was significantly lower in the DL3 arm
- Fevers
- Fevers were seen across all dose levels with different severity and duration within the first 30 days posttransplant
- Fevers recorded in DL2 did not significantly differ from DL1
- Fever was higher and lasted longer in DL3 compared with other dose levels
- Engraftment syndrome was observed in two patients in DL3 with fever, rash, and elevated transaminase levels; however, the events were mild in severity and resolved without intervention
Conclusion
These findings indicated that de-escalation of PTCy (DL2, 25 mg/kg/day given in Days 3−4, or DL3, 25 mg/kg/day given in Day 4 posttransplant) may provide a feasible and effective approach in preventing severe aGvHD. Engraftment appeared to be more rapid compared with the standard dosing (DL1, 50 mg/kg/day given in Days 3−4) and early posttransplant toxicity was less severe.
DL2 was more consistent in early engraftment and resulted in earlier resolution of posttransplant fevers compared with DL3. Therefore, DL2 is currently under investigation in a phase II expansion cohort. Further evaluation in a larger patient sample and longer follow-up is warranted to investigate the superiority of lower PTCy dosing over the current standard dosing.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

