All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Considerations in the treatment of aGvHD
In a recent publication from the American Society of Hematology Education Program,1 Newell and Holtan discussed important factors to consider during the treatment of acute graft-versus-host disease (aGvHD), including risk-adapted treatment approaches and areas where treatment could be improved. The authors also highlighted the importance of considering the severity of patient symptoms, the possibility of alternative diagnoses and provision of supportive care before starting first-line therapy with corticosteroids. If a patient has persistent or recurring symptoms that occur after the initial treatment of aGvHD, then it is important to consider other contributors such as medication side effects, tissue damage, infections, and malabsorption, before starting second-line therapy.1
Visual abstract
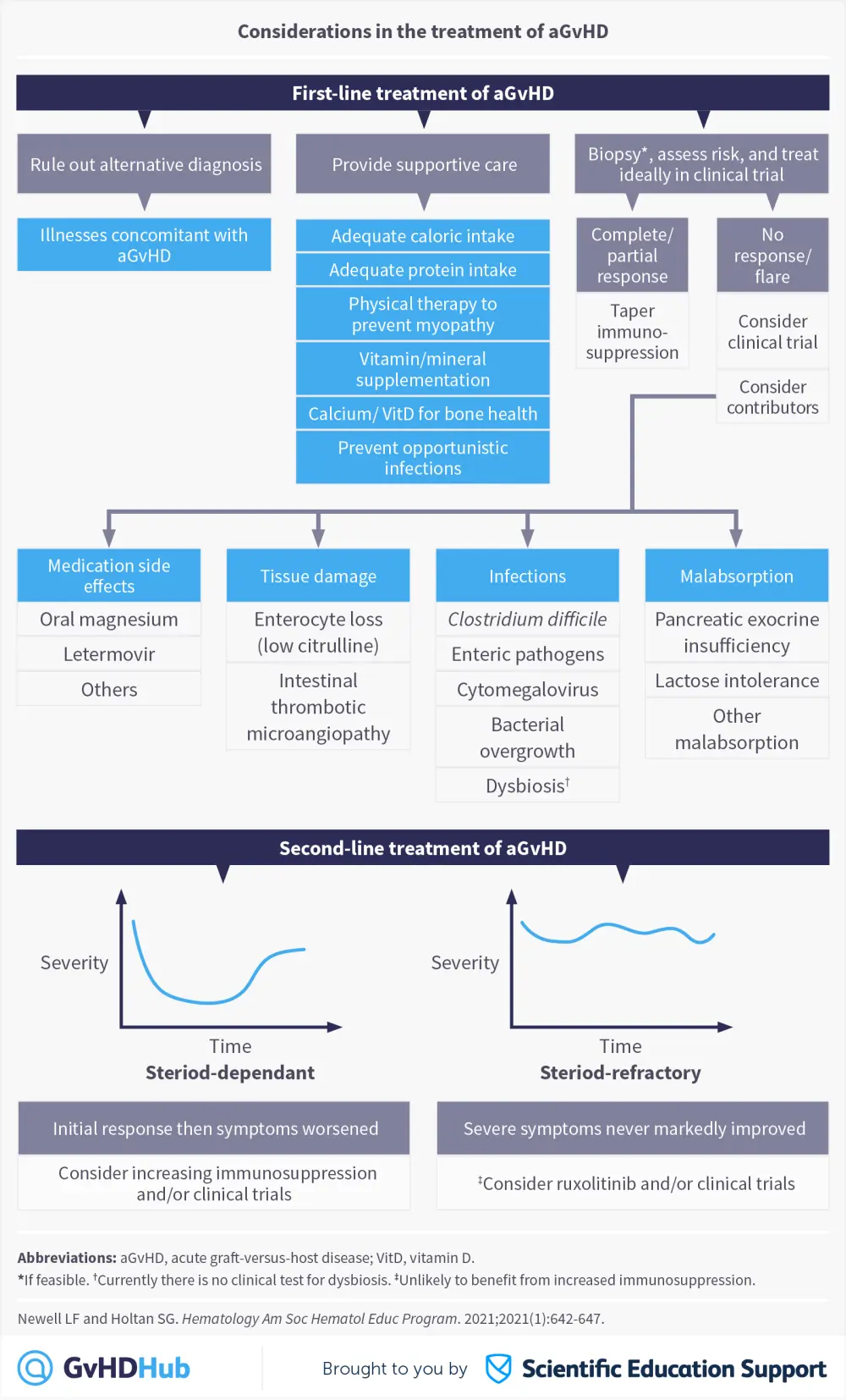
Conclusion
Increasing immunosuppression may not be the best second-line treatment for all patients with worsening symptoms of aGvHD. Although ruxolitinib is the only therapy approved by the U.S Food and Drug Administration (FDA) for the treatment of steroid-refractory aGvHD, it may not be suitable in all patients, especially those who already have infections, as this is a common side effect of the drug.2
To improve outcomes for patients with aGvHD, several questions need to be answered through additional clinical trials, such as:
- How can we distinguish which patients need different modes of supportive care, e.g., remediation of dysbiosis versus tissue damage?
- To achieve maximal mucosal healing, how long should adjunct repair-based therapies such as urinary-derived human chorionic gonadotropin/epidermal growth factor (uhCG/EGF) be continued?
- What other targets of aGvHD (e.g., the endothelium) should be treated?
Biomarker studies may provide an answer to some of these questions and have the potential to be used as a guide to taper immunosuppression.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

