All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Combination of myeloablative conditioning with regulatory and conventional T-cell immunotherapy for AML
The most effective treatment for high-risk acute myeloid leukemia (AML) is allogeneic hematopoietic stem cell transplantation (HSCT). Nevertheless, only 40–50% of patients become long-term survivors with no relapse and/or chronic graft-versus-host disease (cGvHD). Recently, HSCT has become more feasible in older patients due to reduced-intensity conditioning regimens. Despite the advances in treatments, relapse remains a key cause of transplantation failure.1
In a study published in Blood Advances by Pierini et al.,1 a combination of myeloablative conditioning with regulatory and conventional T-cell immunotherapy was investigated for patients with AML. The findings from this study are summarized here.
Study design
The phase II clinical trial (NCT03977103), conducted in Italy, included 50 patients with AML. The patients were aged <75 years (range, 20–65 years) with an ECOG status ≤2 and the availability of an HLA-matched donor family member.
- The primary endpoint was 2-year moderate/severe GvHD/relapse-free survival.
- Secondary endpoints were the incidence of full donor engraftment, cumulative incidence of Grade ≥2 acute GvHD (aGvHD), cGvHD, non-relapse mortality (NRM), and relapse.
The conditioning regimen was total body irradiation (TBI) in patients ≤50 years of age and total marrow/lymphoid irradiation (TMLI) for patients aged 51–65 years or unfit for TBI. Patients received an age-adapted myeloablative conditioning regimen based on TBI, either fractionated (nine fractions delivered twice a day for 4.5 days; total dose, 13.5 Gy) or a single dose of 8 Gy for those aged <50 years or based on TMLI for those aged 51–65 years. TBI or TMLI were followed by thiotepa (5–10 mg/kg), fludarabine (150–200 mg/m2), and cyclophosphamide (30 mg/kg) (see Figure 1).
Figure 1. Transplantation schema*
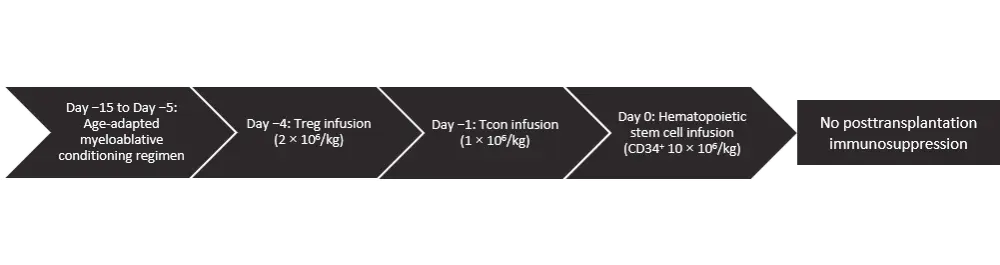
*Figure adapted from Pierini et al.1
Results
Baseline characteristics1
Five patients had favorable risk, 22 intermediate risk, and 20 adverse genetic risk leukemia (see Table 1). Measurable disease at transplantation by cytogenic, immunophenotypic, and/or molecular analyses was observed in 33 patients. Eight patients underwent transplantation with active disease and 42 while in hematologic complete remission.
Table 1. Baseline characteristics*
|
AML, acute myeloid leukemia; CR, complete remission; DRI, Disease Risk Index; HCT-CI, Hematopoietic Cell Transplantation–Specific Comorbidity Index; HSCT, hematopoietic stem cell transplantation; MRD, measurable residual disease; NA, not applicable; PIF, primary induction failure; TBI, total body irradiation; TMLI, total marrow/lymphoid irradiation. |
||||
|
Characteristic |
TBI based |
TMLI based |
Total |
p value |
|---|---|---|---|---|
|
Sex, female |
11 |
13 |
24 |
0.87 |
|
Median age, years (range) |
33 (20–50) |
56 (38–65) |
53 (20–65) |
0.01 |
|
Genetic stratification at diagnosis (%) |
|
|
|
|
|
Other risk factors (%) |
|
|
|
|
|
Disease status at HSCT (%) |
|
|
|
|
|
DRI (%) |
|
|
|
|
|
HCT-CI risk score (%) |
|
|
|
|
GvHD, NRM, relapse, and survival
- Grade ≥2 aGvHD developed in 15 patients (cumulative incidence, 33%; 95% confidence interval [CI] 30–35) and 12 of 15 patients developed Grade 3–4 aGvHD.
- aGVHD occurred in three patients from the TBI group and 12 from the TMLI group (TBI: cumulative incidence, 16%; 95% CI, 13–18 vs TMLI: cumulative incidence, 41%; 95% CI, 39–43; [p = 0.05]).
- The median onset of aGvHD was 41 days (range, 23–69).
- Twelve of the 15 patients with aGvHD did not progress to moderate/severe cGvHD, were alive, and did not require prolonged immunosuppressive therapy. Of the remaining three patients, two died due to aGvHD and one due to leukemia relapse.
- NRM occurred in ten patients (cumulative incidence, 21%; 95% CI, 20–23). There was no difference in NRM between the two treatment groups.
- Two patients relapsed (cumulative incidence, 4%; 95% CI, 3–6) at a median follow-up of 34 months (range, 5–72 months).
- GvHD/relapse-free survival was 48% (95% CI, 44–51), and moderate/severe cGvHD/relapse-free survival was 75% (95% CI, 71–78).
- Survival was not affected by irradiation protocol, i.e., TBI vs TMLI, or by adverse genetic risk at diagnosis.
Engraftment and immune rebuilding
- Median time to platelet recovery was 17 days (range, 14–72 days).
- All patients except one achieved full donor-type engraftment and an absolute neutrophil count >0.5 × 109/L in a median time of 13 days (range, 8–23 days).
- The median duration of CD+ and CD8+ attaining 100/µL was 45 days (range, 29–95 days) and 27 days (range, 19–60 days), respectively, and attaining 200/µL was 74 days (range, 39–81 days) and 50 days (range, 25–81 days), respectively.
Adverse events
The majority of conditioning regimen-related adverse events (AEs) were Grade 1–2. One patient developed Grade 5 central nervous system toxicity and died. Two patients died of septic shock. Febrile neutropenia was experienced by all patients during the aplastic phase. There were 19 cases of sepsis observed in a total of 65 febrile neutropenia events. Organ-specific toxicities were reported in all patients (Table 2). Twenty-two of 50 patients had cytomegalovirus reactivations or cytomegalovirus detection in tissue biopsies.
Table 2. Organ-specific toxicities*,†
|
CNS, central nervous system; TBI, total body irradiation; TMLI, total marrow/lymphoid irradiation. †Data from Pierini et al.1 |
||||||||
|
|
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
||||
|---|---|---|---|---|---|---|---|---|
|
TBI |
TMLI
|
TBI
|
TMLI |
TBI |
TMLI |
TBI |
TMLI |
|
|
Oral cavity |
68 |
81 |
32 |
19 |
— |
— |
— |
— |
|
CNS |
— |
6 |
— |
6 |
— |
— |
— |
3 |
|
Hepatic |
5 |
10 |
— |
3 |
16 |
6 |
— |
— |
|
Gastric |
95 |
94 |
5 |
6 |
— |
— |
— |
— |
|
Intestinal |
58 |
68 |
42 |
32 |
— |
— |
— |
— |
|
Renal |
— |
— |
— |
— |
— |
— |
— |
— |
|
Pulmonary |
32 |
35 |
5 |
— |
— |
— |
— |
— |
|
Bladder |
— |
6 |
5 |
— |
— |
— |
— |
— |
|
Cardiac |
32 |
10 |
5 |
— |
— |
— |
— |
— |
Conclusion
The study achieved notably low leukemia relapse and cGvHD rates in patients with AML. This was achieved even though the majority of patients were aged 50–65 years, suggesting that T cell-depleted haploidentical HSCT with TMLI-based conditioning and Treg/Tcon immunotherapy may be applied in patients who are not eligible for standard myeloablative conditioning regimens due to their age. The findings from this study need to be challenged further in a larger, multicenter trial.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

