All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Case study | Therapy-refractory chronic GvHD
During the GvHD Hub Steering Committee meeting, key opinion leaders met to discuss a case study on the diagnosis and management of a patient with therapy-refractory chronic GvHD (cGvHD). This recorded discussion was chaired by Hildegard Greinix, and featured Yi-Bin Chen, Mohamad Mohty, Attilio Olivieri, Arnon Nagler, and Florent Malard.
Case study | Therapy-refractory chronic GvHD
Case study
Hildegard Greinix presented a case study, including the treatment pathways and progression of symptoms, for discussion by the members of the steering committee. This case presentation is outlined in Figure 1.
Figure 1. Case study outline
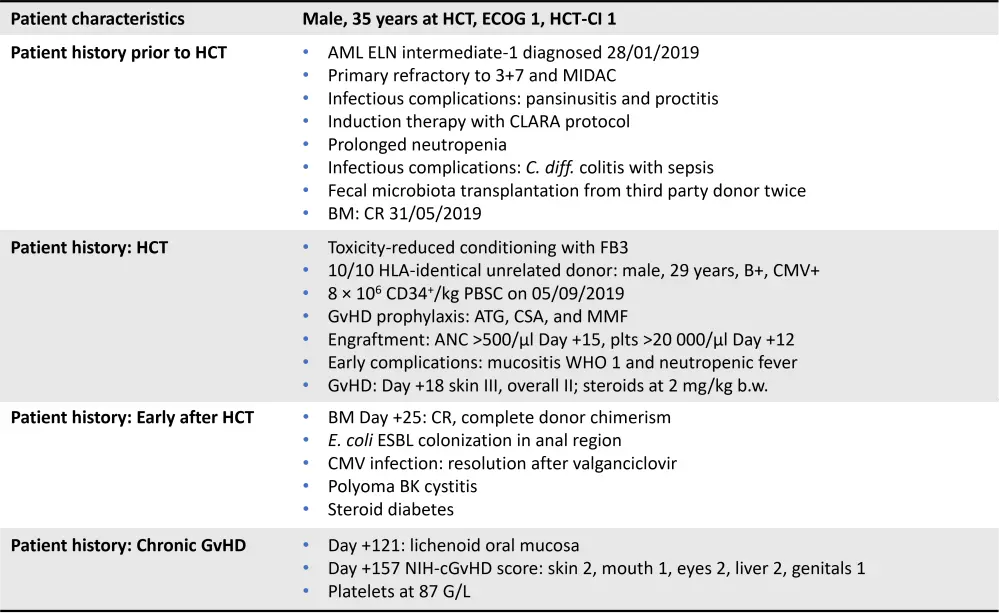
AML, acute myeloid leukemia; ANC, absolute neutrophil count; ATG, antithymocyte globulin; BM, bone marrow; b.w., body weight; CSA, cyclosporine A; CMV, cytomegalovirus; CR, complete response; ECOG, Eastern Cooperative Oncology Group; ELN European Leukemia Network; FB3, fludarabine busulfan 3 days; HCT-CI, hematopoietic cell transplant-comorbidity index; HLA, human leukocyte antigen; MIDAC, mitoxantrone and cytarabine; MMF, mycophenolate mofetil; PBSC, peripheral blood stem cells; plts, platelets; WHO, World Health Organization.
Following hematopoietic cell transplant (HCT), this patient was treated with steroids and cyclosporine, both of which were tapered when acute GvHD resolved. The patient later presented with lichenoid oral mucosa and the National Institute of Health (NIH)-cGvHD scores outlined in Figure 1, suggesting moderate cGvHD. The treatments indicated for this severity of GvHD are outlined in Figure 2.
Figure 2. Indications for treatment of moderate/severe cGvHD*

cGvHD, chronic graft-versus-host disease; GvL, graft-versus-leukemia.
*Adapted from Wolff, et al.1
Following cGvHD diagnosis, this patient was given a combination of steroids, cyclosporine, and extracorporeal photopheresis (ECP) due to their higher risk status. Four months post treatment initiation, the patient only had eye involvement and steroids were tapered. ECP was also discontinued, leaving only cyclosporine as the maintenance therapy.
Following steroid tapering, symptoms of lung involvement presented, including a cough and dyspnea. Pulmonary function tests revealed a reduced forced expiratory volume of 25%, indicating a severe obstruction using the NIH scoring system and a diagnosis of severe cGvHD.2
Discussion
In this historical case, ruxolitinib was not available. However, in a modern case, ruxolitinib could be indicated to inhibit the production of proinflammatory cytokines and reduce symptoms of GvHD. Ruxolitinib may be applied alongside or in place of ECP at first diagnosis of acute GvHD post HCT.
There is a question around when a lung biopsy is appropriate after a severe cGvHD diagnosis. A biopsy could provide more histological data that may inform management plans; however, this invasive procedure is not currently routinely performed on patients with cGvHD and lung involvement.
In this case, the addition of lung involvement and severe cGvHD diagnosis occurred after the tapering of steroids, which poses the question of determining if this manifestation is steroid refractory/dependent. However, to establish this it would be necessary to readminister and taper steroids. This could be detrimental to the patient and is therefore not an appropriate option to obtain a diagnosis. Considering multiple lines of therapy were delivered, and that it is impossible to make a steroid refractory diagnosis, this patient was considered to be therapy refractory by the GvHD Hub Steering Committee.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?


 Mohamad Mohty
Mohamad Mohty Attilio Olivieri
Attilio Olivieri Florent Malard
Florent Malard Arnon Nagler
Arnon Nagler Yi-Bin Chen
Yi-Bin Chen Hildegard Greinix
Hildegard Greinix