All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
Non-restrictive diet post-HSCT: Preliminary analysis of phase III prospective trial data presented at EHA2022
Despite the widespread use of restrictive diets post-hematopoietic stem cell transplantation (HSCT) to reduce infection rates, for example low microbial diets, no prospective evidence exists to validate their use.1 In fact, some observational studies suggest a possible link between restrictive diets and higher infection rates.1
At the European Hematology Association (EHA) 2022 Congress, Stella1 presented preliminary findings of their prospective, multicenter, phase III NEUTRODIET trial, which is studying the effects of diet on infection rates during neutropenia in patients undergoing autologous (auto)- or allogeneic (allo)‑HSCT. We are pleased to provide a summary of this presentation here.
Given the significant morbidity and mortality associated with infections during neutropenia and graft-versus-host-disease (GvHD), as well as the potential for restrictive diets to significantly impact quality of life, the aim of the study was to prospectively compare restrictive and non-restrictive diets during neutropenia posttransplant. Furthermore, given the known correlation between reduced oral intake after allo-HSCT and increased rates of GvHD, Stella and colleagues postulate that these restrictive diets may also contribute to GvHD given their limiting and less palatable nature.
Study design
The inclusion criteria for the study were patients undergoing either an allo- or auto‑HSCT, and exclusion criteria are shown in Figure 1. Eligible patients were randomized to either Arm A or Arm B depending on if they received a protective diet or non-restrictive diet (NRD), respectively. Stratification of the patients who underwent allo‑HSCT was also performed.
The primary endpoint was to demonstrate the absence of significant differences in infections and deaths during the period of neutropenia between the two arms. Secondary endpoints included assessment of gastrointestinal (GI) infections, fever of unknown origin, body weight change, length of hospital stay, 30-day estimated overall survival, and cumulative incidence of acute GvHD (aGvHD).
Figure 1. Study design*
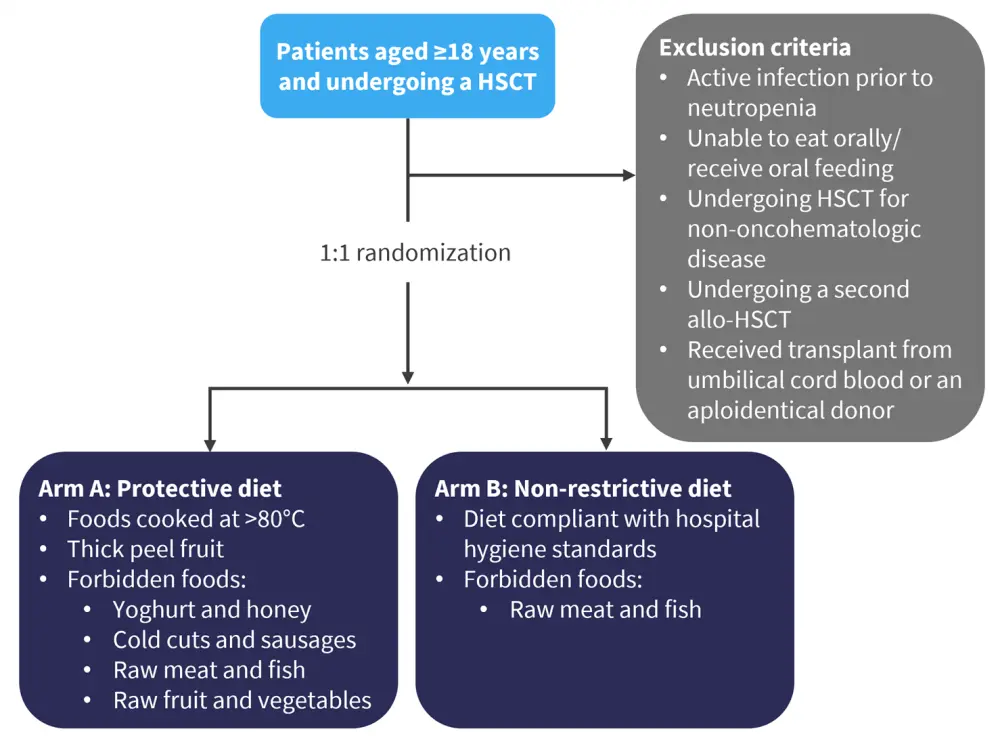
HSCT, hematopoietic stem cell transplant.
*Adapted from Stella, et al.1
Baseline characteristics
Of the 244 patients enrolled, preliminary results of the first 200 were presented. Baseline characteristics of all patients are shown in Table 1. There were no significant differences between the two study arms. The median age of patients was 56 years, with the most common disease types being aggressive lymphoma and multiple myeloma.
Table 1. Baseline patient characteristics of all patients*
|
Allo-HSCT, allogeneic hematopoietic stem cell transplant; AML, acute myeloid leukemia; auto‑HSCT, autologous hematopoietic stem cell transplant; CR, complete response; N/A, not applicable; PD, progressive disease; PR, partial response; SD, stable disease. |
||
|
Characteristic, % (unless otherwise stated) |
Protective diet |
Non-restrictive diet |
|---|---|---|
|
Female |
39 |
42 |
|
Median age (range), years |
56 (26–71) |
56 (22–71) |
|
Disease type |
|
|
|
Aggressive lymphoma |
38 |
39 |
|
Indolent lymphoma |
10 |
5 |
|
Multiple myeloma |
40 |
38 |
|
AML |
3 |
3 |
|
Other |
8 |
14 |
|
Number of previous therapy lines |
|
|
|
Median (range), n |
1 (1–3) |
1 (1–3) |
|
≥2 |
50 |
50 |
|
Disease status at enrollment |
|
|
|
CR |
60 |
51 |
|
PR |
31 |
33 |
|
SD |
1 |
7 |
|
PD |
2 |
5 |
|
N/A |
5 |
5 |
|
Antimicrobial prophylaxis |
|
|
|
Antiviral |
94 |
99 |
|
Antibacterial |
65 |
67 |
|
Antifungal |
94 |
95 |
|
Type of transplant |
|
|
|
Auto-HSCT |
78 |
79 |
|
Allo-HSCT |
22 |
21 |
|
Neutropenia duration (range), days |
6 (3–20) |
5 (3–18) |
Results
- No significant differences in infection rate were seen between the two study arms.
- Infections Grade >2 were slightly decreased in the protective diet arm compared with the NRD arm (relative risk [RR], 0.9; 95% confidence interval [CI], 0.6–1.2; Figure 1)
- Incidence of fever of unknown origin, febrile neutropenia, sepsis, and pneumonia were not significantly different.
- A higher incidence of documented GI infection (defined as abdominal symptoms in combination with microbiological isolation of a pathogen by stool test or GI mucosa biopsy) was seen in the protective diet arm compared with the NRD arm (RR, 3.7; 95% CI, 1.2–12.2; p = 0.03; Figure 1).
Figure 2. Incidence of total and GI infections*
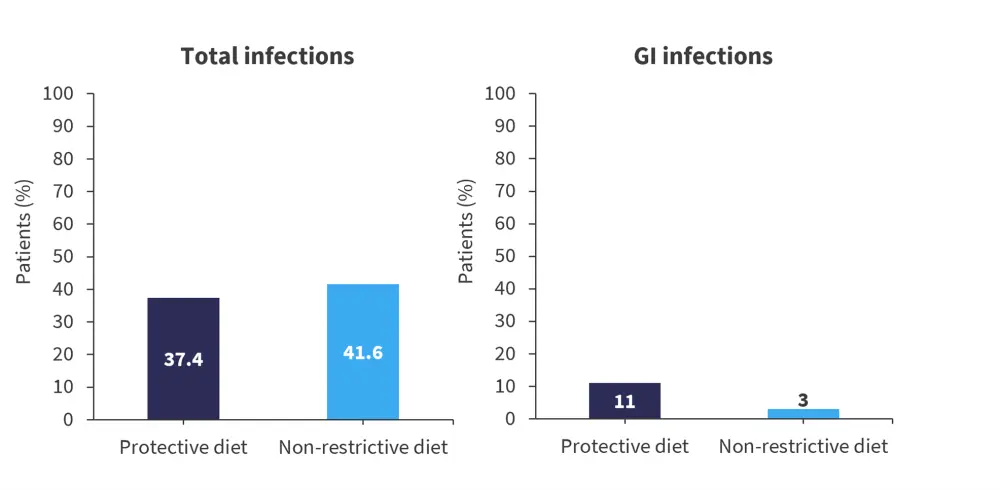
GI, gastrointestinal.
*Adapted from Stella, et al.1
- No significant differences in the incidences of GI infection without microbiological isolation of a pathogen or mucositis were seen between the two study arms.
- No significant differences were seen in body weight variations from admission to discharge, hospitalization length, use of parenteral nutrition (both rate of use and duration), and serum albumin variation.
- One death occurred in the NRD arm, secondary to cytokine release syndrome.
Patients undergoing allo-HSCT
For patients receiving allo-HSCT, baseline characteristics were well balanced between the two arms (Table 2). No significant difference was seen in the incidence of aGvHD Grade ≥2 (protective diet arm, 24%; NRD arm, 27%; RR, 0.9; 95% CI, 0.3–2.3; p > 0.99), all grades of aGvHD, or GI aGvHD.
Table 2. Baseline characteristics of patients undergoing allo-HSCT*
|
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; ATG, antithymocyte globulin; CsA-MMF-PTCy, cyclosporine-A, mycophenolate mofetil, posttransplant cyclophosphamide; CsA-MTX, cyclosporine-A, methotrexate; GvHD, graft-versus-host-disease; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; HLA, human leukocyte antigens; MAC, myeloablative conditioning; RIC, reduced intensity conditioning. |
||
|
Characteristic, % (unless otherwise stated) |
Protective diet |
Non-restrictive diet |
|---|---|---|
|
Disease type |
|
|
|
Lymphoma |
67 |
50 |
|
AML |
14 |
14 |
|
Other |
19 |
36 |
|
Donor |
|
|
|
Related |
43 |
41 |
|
Unrelated |
67 |
69 |
|
HLA matching |
|
|
|
10/10 |
57 |
50 |
|
9/10 |
24 |
27 |
|
<9/10 |
19 |
23 |
|
Conditioning |
|
|
|
MAC |
33 |
50 |
|
RIC |
67 |
50 |
|
GvHD prophylaxis |
|
|
|
CsA-MTX |
71 |
81 |
|
CsA-MMF-PTCy |
29 |
19 |
|
ATG |
52 |
63 |
|
HCT-CI |
|
|
|
0 |
14 |
23 |
|
1–2 |
62 |
54 |
|
≥3 |
24 |
23 |
Conclusion
The preliminary results of this prospective trial presented by Stella suggest that restrictive diets after auto- or allo-HSCT do not offer any benefit in reducing rate of infection, GvHD, or deaths. Given these diets may place unnecessary burden on patients and potentially reduce their quality of life, the full results of this study will be awaited with interest and will include analyses of patient satisfaction and of the gut microbiome.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

