All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The gvhd Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the gvhd Hub cannot guarantee the accuracy of translated content. The gvhd and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The GvHD Hub is an independent medical education platform, sponsored by Medac and supported through grants from Sanofi and Therakos. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View GvHD content recommended for you
A phase II trial of ALXN1007, a C5a complement inhibitor, for use in lower GI-aGvHD
Acute graft-versus-host disease (aGvHD) commonly occurs after allogeneic hematopoietic stem cell transplant (HSCT) and, in 60% of patients, it will involve the lower gastrointestinal tract (LGI).1 Generally, first line therapy for aGvHD is corticosteroids; however, the LGI is less responsive than other organs to steroid treatment and previous clinical trials have demonstrated that use of immunosuppressants does not improve outcomes.1
Preclinical studies have suggested that complement C3 and C5 may be associated with GvHD, with inhibition of C5a in T-cell C5aR knockout shown to prevent the disease. Therefore, novel LGI aGvHD therapies which can inhibit the complement cascade may be beneficial, offering an alternative therapy option for steroid-refractory patients.
One therapy that targets the complement cascade is ALXN1007, an anti-C5a monoclonal antibody that has previously shown positive results in preclinical and phase I studies, which may act as a complete agonist of C5a. Complement C5a drives inflammation through phagocyte recruitment. Here, we report the results of a phase IIa non-randomized study (NCT02245412) evaluating the safety and efficacy of ALXN1007 in patients with aGvHD across three cohorts.1
Study design1
This study enrolled a total of 25 patients in three sequential cohorts who received ALXN1007 on three separate dosing schedules. The primary endpoints of the study included incidence and severity of adverse events and the LGI aGvHD overall response rate at Day 28. Secondary endpoints included LGI aGvHD response rates at Day 56. The study design is shown in Figure 1.
Figure 1. Study design*
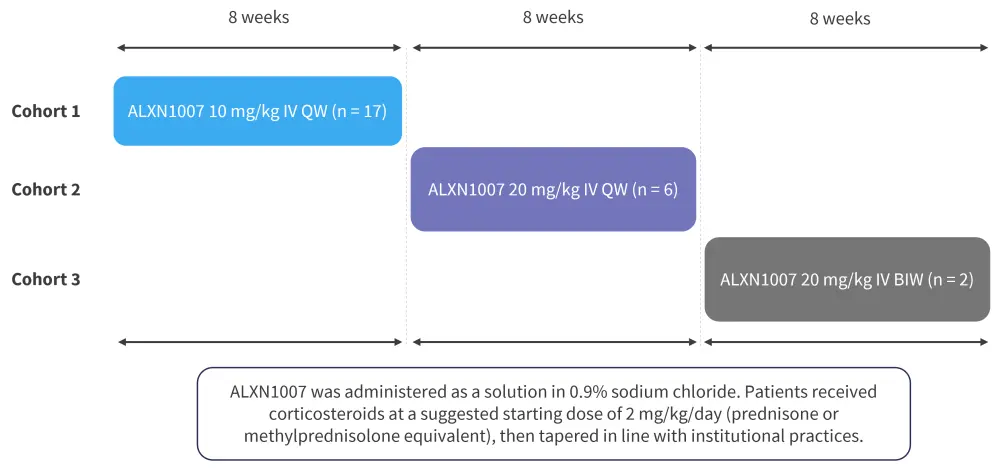
BIW, twice weekly; IV, intravenous; QW, once weekly.
*Adapted from Mehta, et al.1
Results1
The median follow-up was 11.3 months in cohort 1, 8.6 months in cohort 2, and 2.7 months in cohort 3. Patient characteristics at baseline are shown in Table 1.
Table 1. Baseline patient characteristics*
|
CNI, calcineurin inhibitor; GvHD, graft-versus-host disease; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplant; LGI, lower gastrointestinal tract; MMF, mycophenolate mofetil; MTX, methotrexate. |
||||
|
Characteristic, % |
Cohort 1 |
Cohort 2 |
Cohort 3 |
Total |
|---|---|---|---|---|
|
Age(at HSCT (range), years |
60 (25–69) |
46 (36–70) |
69 and 72 |
60 (25–72) |
|
Gender |
|
|
|
|
|
Female |
47 |
33 |
50 |
44 |
|
Male |
53 |
67 |
50 |
56 |
|
Donor |
|
|
|
|
|
HLA- |
24 |
17 |
0 |
20 |
|
HLA- |
47 |
50 |
100 |
52 |
|
Cord blood |
24 |
17 |
0 |
20 |
|
HLA-mismatched related |
6 |
17 |
0 |
8 |
|
GvHD prophylaxis |
|
|
|
|
|
CNI |
29 |
0 |
50 |
24 |
|
CNI + MTX |
18 |
17 |
0 |
16 |
|
CNI + MMF |
53 |
50 |
0 |
48 |
|
Median time from HSCT to LGI GvHD (range), days |
50 (30–184) |
38 (16–76) |
30 and 75 |
48 (16–184) |
|
Median time from HSCT to ALXN1007 treatment (range), days |
48 (26–181) |
37.5 (17–73) |
29 and 73 |
48 (17–181) |
Safety
Of the 25 patients enrolled, 17 completed 8 weeks of treatment. Of these patients, all experienced at least one adverse event (AE). The rate of AEs, rate of infections, and most common AEs is shown in Figure 2.
Figure 2. AE rate and infection rate for all cohorts*
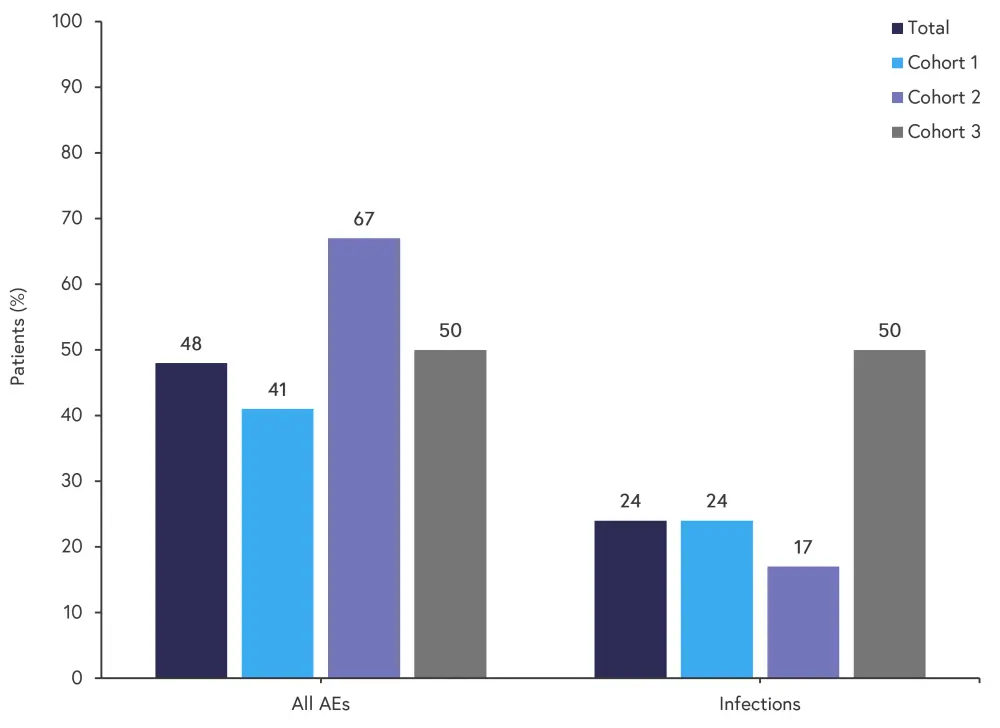
AE, adverse event.
*Adapted from Mehta, et al.1
Efficacy
At Day 56, 15 of 24 patients had achieved a complete response. Responses at Day 28 and Day 56 are shown in Figure 3.
Figure 3. Responses at Day 28 and Day 56*
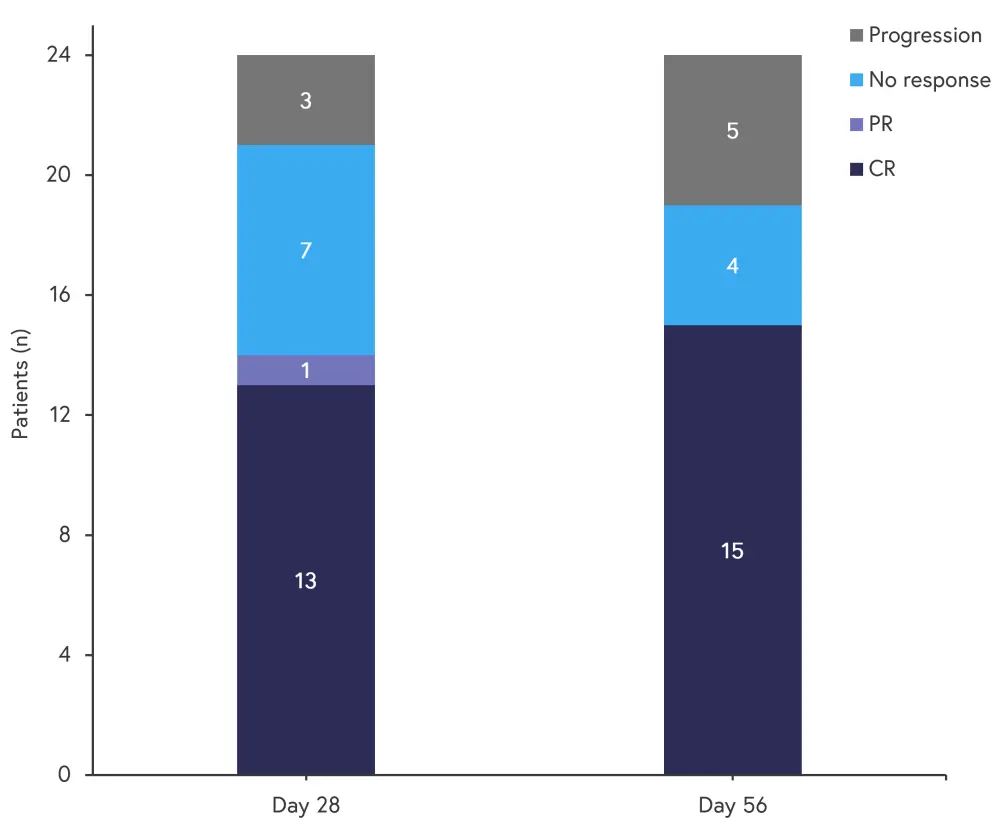
CR, complete response; PR, partial response.
*Adapted from Mehta, et al.1
Survival rates and the rate of non-relapse mortality at 12 months are shown in Figure 4.
Figure 4. Survival and mortality rates at 12 months*
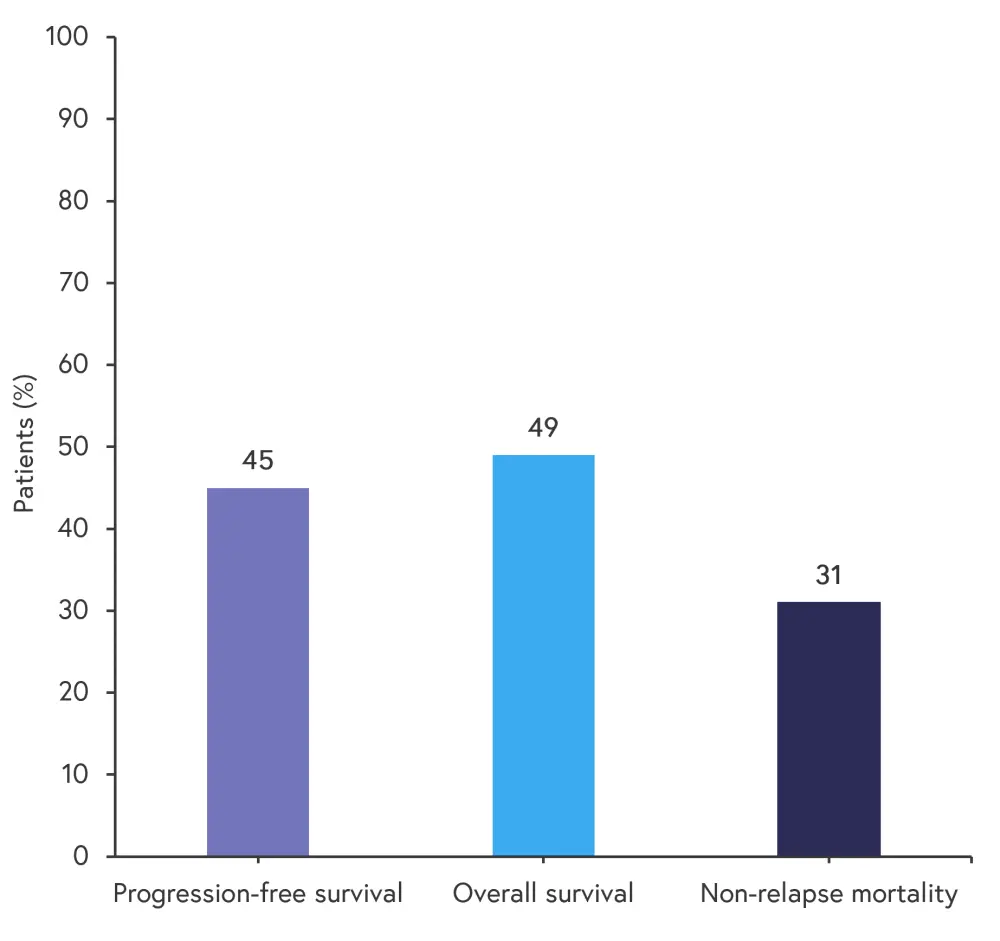
*Adapted from Mehta, et al.1
The role of complement
In this study, most patients were found to have normal C3 and C4 levels at baseline (71% and 83% of patients, respectively). In addition, the levels of C3, C4, and C5 at baseline did not appear to be associated with GvHD severity. Although these data may not be fully representative of complement pathway activity, the findings suggest that systemic activation of complement may not occur in all patients with LGI-aGvHD.
Conclusion
This phase II study showed that ALXN1007 can be safely used in patients with LGI-aGvHD in combination with corticosteroids.1 The rate of AEs was similar to previous AE rates seen in patients with aGvHD treated with corticosteroids alone.1 Overall, 63% of patients achieved a complete response at Day 56, with almost 50% of patients surviving to 12 months; these positive results warrant further clinical trials with ALXN1007 in patients with LGI-aGvHD. However, it should be noted that the findings of this study are limited by the small sample size of each cohort.1
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
Which consideration most strongly guides your decision to escalate therapy in SR-aGvHD?

